Preparation of Intermetallic Nanoparticles
A disadvantage of the unsupported model systems is the low specific surface area (~ 0.1 m2g-1), resulting in a low specific catalytic activity. Since an increase in activity while retaining the selectivity by top-down methods could not be realised, the syntheses of nanoparticulate Ga-Pd and Pd-Zn intermetallic compounds were developed.1,2
The synthesis of nanoparticulate Ga-Pd compounds was achieved by simultaneous reduction of GaCl3 and Pd(acac)2 with superhydride in THF under inert gas conditions. Since the particles were initially unsupported after synthesis, i.e. in suspension, and no further insoluble byproducts are included, the nanoparticles can be fully characterised. The phases and crystal size were verified by XRD, TEM investigations revealed single-crystallinity of the particles, whose size was consistent with X-ray results. The particle size distributions were determined by means of a disc centrifuge and the resulting specific surface area (up to 12 m2g-1) was verified by BET measurements.
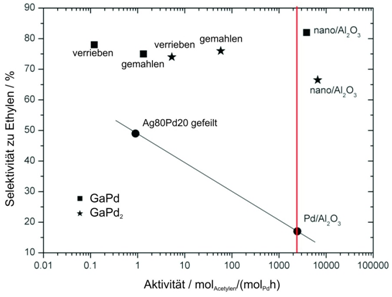
After characterisation the nanoparticles were deposited on Al2O3 for the catalytic reaction test. In the figure, it can clearly be seen that the specific activity of the nanosized catalysts is higher than that of unsupported bulk samples by several orders of magnitude. In addition, the excellent selectivity could be maintained (see. Fig. 1).
|
|
Figure 1: selectivity and activity of the Pd-Ga intermetallic compounds compared to Pd20Ag80 and Pd/Al2O3. |
- M. Armbrüster, G. Wowsnick, M. Friedrich, M. Heggen, R. Cardoso-Gil, J. Am. Chem. Soc. 133, 2011, 9112. doi: 10.1021/ja202869d
- Y. Luo, Y. Sun, U. Schwarz, M. Armbrüster, Chem. Mater. 24, 2012, 3094. doi: 10.1021/cm3018192