Research: Catalytic hydrogenations and bifunctional catalysts
I. Catalytic homogeneous hydrogenation with 3d metal complexes
The group is interested in the development of efficient transition metal-based catalysts for catalytic dihydrogen (H2) activation and hydrogen transfer reactions based on readily available 3d elements.
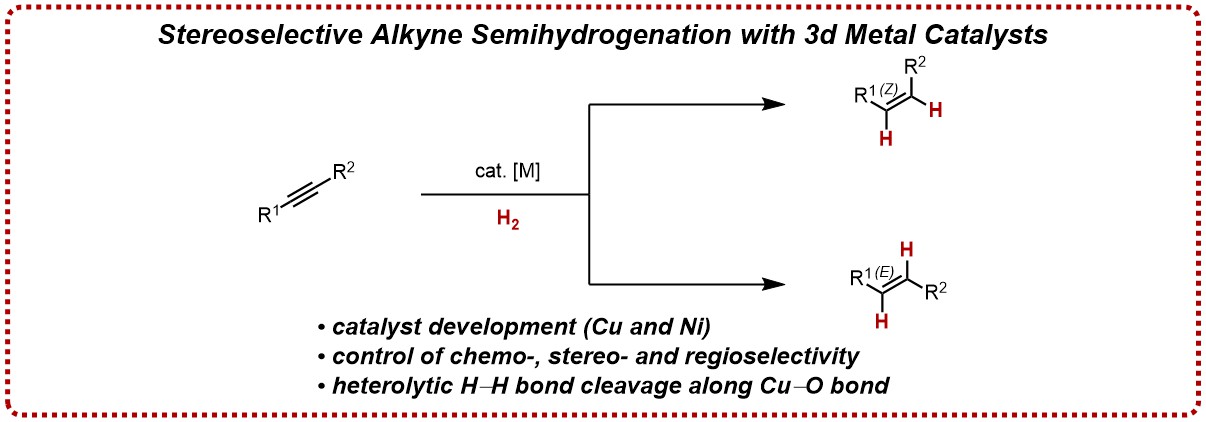
In this vein, we are exploring copper(I)/N-heterocyclic carbene (NHC) complexes for heterolytic H-H bond cleavage and downstream chemo- and stereoselective alkyne semihydrogenations. Key findings have been that NHC ligands enable highly Z-selective processes as well as the suppression of any overreduction to the corresponding alkanes.
See for example: Chem. Eur. J. 2015, 21, 15934; Org. Biomol. Chem. 2016, 14, 10660; Synthesis 2017, 49, 2470; Tetrahedron 2017, 73, 5023; Synlett 2019, 30, 783.
For a general review regarding ligand design of NHCs and "tethered" NHC ligands, see: Eur. J. Org. Chem. 2017, 29, 4206.
Departing from copper(I) hydride chemistry from H2, our group has developed a simple and practical protocol to effect the synthetically much more challenging E-selective alkyne semihydrogenation based on a simple and readily available nickel complex. In this method, a wide variety of internal alkynes can be converted with high stereoselectivity to E-alkenes with a remarkably high tolerance of functional groups.
See for example: Chem. Eur. J. 2020, 26, 1597.
Furthermore, we have been able to show that via this approach, the classic “copper hydride catalysis” can be made accessible, using only H2 as terminal reducing agent. In this vein, we have shown that conjugate reductions of enoates and enamides can be effected by copper(I)/NHC complexes as catalysts. In a further extension of this work, we can show that a hydride transfer from H2 to allylic acceptors is possible, resulting in an “allylic reduction”. This work shows that catalytic H2 activation and hydride transfer is possible in the presence of alkenes, which do not undergo catalytic hydrogenation to the corresponding alkanes. This underscores one of the main motivations of our work – the control of chemoselectivity by catalyst and ligand design.
See for example: Org. Lett. 2016, 18, 2455; Chem. Commun. 2017, 53, 11686; Chem. Eur. J. 2019, 25, 985.

For a general review on copper-catatalyzed homogeneous hydrogenation, see: Homogeneous Hydrogenation with Copper Catalysts (Book Chapter) in Homogeneous Hydrogenation with Non-Precious Catalysts, Wiley, Weinheim, 2019.
II. Copper(I)-catalyzed transfer hydrogenation
While our main focus lies on the use of cheap and readily available dihydrogen (H2), we have shown that our copper(I)/NHC catalysts can also be employed in a transfer hydrogenation setting (alkyne semihydrogenation as well as conjugate reduction). In these protocols either ammonia borane or simple alcohols can be used as H2 equivalents. The overall transformation mirror the high selectivity of the hydrogenative variants, however, they do not require high pressure equipment, making the protocols much simpler.
See for example: Chem Commun. 2017, 53, 732; Chem. Commun. 2019, 55, 13410.

III. Ligand design – bifunctional catalysts
The group is exploring the combination of transition metal complexes and organocatalysts within one molecular framework to fundamentally study and understand bifunctional catalysis. In this manner, new reactivity as well as new means to control and predict selectivity in homogeneous catalysis can be studied.
We have recently shown that the combination of copper/N-heterocyclic carbene complexes and hydrogen-bond organocatalysts enables the realization of hitherto unknown reactivity of copper hydride complexes. In this vein, we are now able to control reactivity by supramolecular interaction, which allows the reduction of formally “hard” electrophiles with the typically “soft” copper hydride reducing agents. This is exemplified by a copper(I)-catalyzed homogeneous hydrogenation of esters to the corresponding alcohols.
See for example: J. Am. Chem. Soc. 2021, 143, 16865; Beilstein J. Org. Chem. 2023, 19, 440.

IV. Homogeneous hydrogenation of polymers
Based on the abovementioned expertise on catalytic hydrogenation of carboxylic acid derivatives with bifunctional catalysts, we are currently exploring the homogeneous hydrogenation of polymers (such as polyesters) with these 3d metal catalysts. Research in this area is carried out in collaboration with the polymer chemistry research group at TU Chemnitz.
