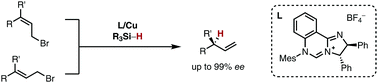Publications
|
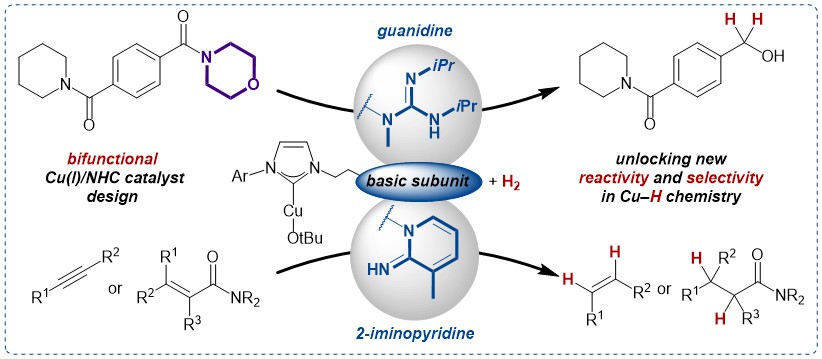
52. G. Pal, M.Voigtländer, D.-I. Tzaras, M. Gorai, J.F. Teichert Enhancing Reactivity and Selectivity of H2-driven Copper Hydride Chemistry with Bifunctional N-heterocyclic Carbene Ligands Bearing Basic Subunits (Synpacts article) Synlett 2025, published online; DOI: 10.1055/a-2694-7263 |
© 2025 Thieme |
|
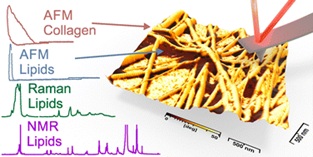
51. M. Dehnert, T. Klose, Y. Pan, D.R.T. Zahn, M. Voigtländer, J.F. Teichert, R. Magerle Triacylglycerols affect the water content and cohesive strength of collagen fibrils Soft Matter 2025, 21, 7917; DOI: 10.1039/D5SM00696A |
© 2025 RSC |
|
50. D.-I. Tzaras, M. Voigtländer, B.M. Zimmermann, T. Rüffer, J.F. Teichert Synthesis of a library of bifunctional N-heterocyclic carbene ligand precursors with hydrogen bond donor subunits
published as preprint: ChemRXiv 2025; DOI: 10.26434/chemrxiv-2025-570l9
|
© 2025 Teichert group |
|
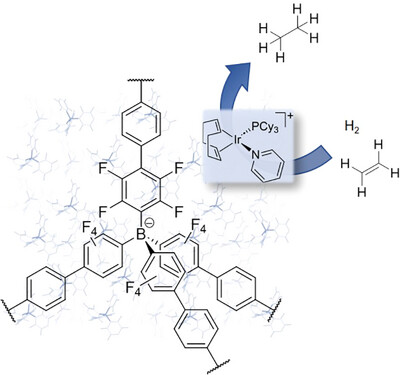
49. M. Gorai, P. Rotering, J. Franzen, F. Dielmann, J.F. Teichert A ligand-assisted proximity effect allows for H2-driven copper hydride chemistry under mild conditions J. Am. Chem. Soc. 2025, 147, 14481. DOI: https://pubs.acs.org/doi/10.1021/jacs.5c01339 published as preprint: ChemRXiv 2024; DOI: 10.26434/chemrxiv-2024-w3lxs |
© 2025 Teichert group |
|
48. R. Sroka, Y. Okhassova, N. Chaoui, M. Trunk, J. Schmidt, S. Fischer-Lang, J.F. Teichert, A. Thomas Weakly Coordinating Anionic Microporous Polymer Networks as Scaffold for Molecular Gas Phase Hydrogenation Catalysts ChemCatChem 2025, 17, e202401595. DOI: https://doi.org/10.1002/cctc.202401595 |
© 2025 Wiley-VCH |
|
47. D.-I. Tzaras, T. Jacquemin, M. Gorai, T. Arndt, B.M. Zimmermann, M. Breugst, J.F. Teichert Site-selective copper(I) catalyzed hydrogenation of amides J. Am. Chem. Soc. 2025, 147, 1867; DOI: https://doi.org/10.1021/jacs.4c14174 published as preprint: ChemRXiv 2024; DOI: 10.26434/chemrxiv-2024-rz1j4 This work was highlighted by Chemistry Views and in Synfacts 2025, 21, 270.
|
© 2025 American Chemical Society |
|
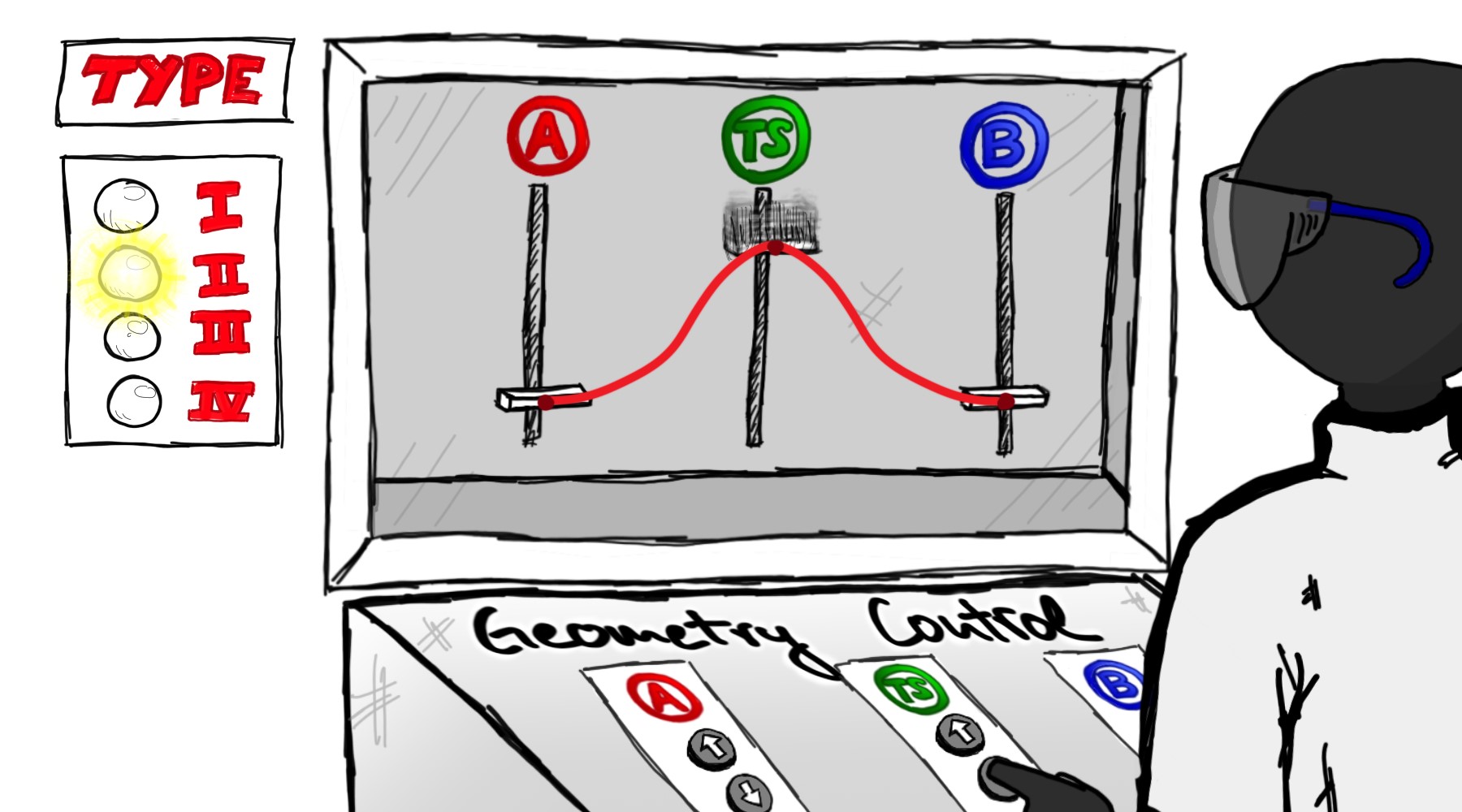
46. P. Saha, T. Tran Ngoc, P. McGonigal, J.F. Teichert Geometry-Controlled Reactivity and Dynamics in Organic Molecules |
© 2024 Teichert group |
|
45. M. Gorai, J.F. Teichert Chiral bifunctional NHC/guanidine ligands for asymmetric hydrogenation Synlett 2024, 35, 989: DOI: 10.1055/s-00000083 as part of the cluster Chemical Synthesis and Catalysis in Germany published as preprint: ChemRXiv 2023; DOI: 10.26434/chemrxiv-2023-k9lwz |
© 2023 Teichert group |
|
44. A. Das, J.F. Teichert Eliminations to form alkenes, allenes and alkynes and related reactions (book chapter) Comprehensive Organic Synthesis, 3rd Ed. Volume 6: Heteroatom Manipulation, Elsevier. DOI: https://doi.org/10.1016/B978-0-323-96025-0.00012-0
|
|
|
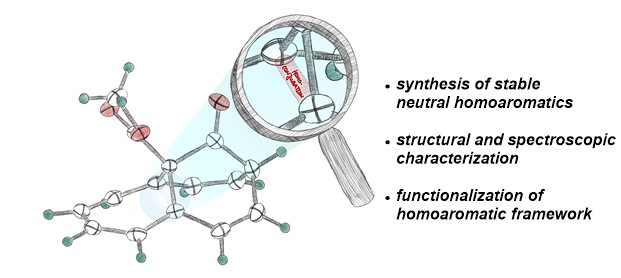
43. T. Tran Ngoc, J. van der Welle, T. Rüffer, J.F. Teichert Synthesis of stable neutral homoaromatic hydrocarbons published as Feature Article in Synthesis 2023, 55, 2658; DOI: 10.1055/s-0042-1751468. published as preprint: ChemRXiv 2023, DOI: 10.26434/chemrxiv-2023-kbtbm |
© 2023 Teichert group |
|
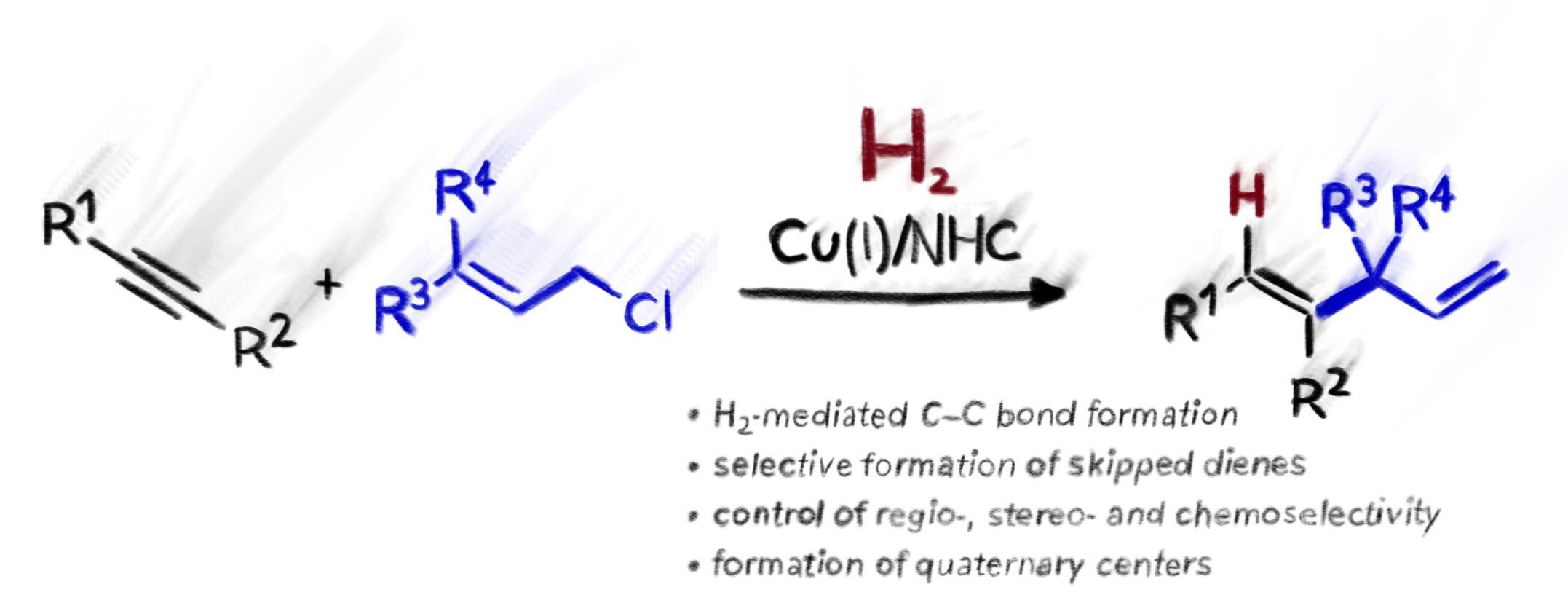
42. L.T. Brechmann, B. Kaewmee, J.F. Teichert, H2-mediated copper catalyzed C-C coupling reactions - selective formation of skipped dienes ACS Catal. 2023, 13, 12634; DOI: 10.1021/acscatal.3c03141. published as preprint: ChemRXiv 2023, DOI: 10.26434/chemrxiv-2023-3w2cn |
© 2023 Teichert group |
|
41. T. Tran Ngoc, N. Grabicki, E. Irran, O. Dumele, J.F. Teichert, Photoswitching neutral homoaromatic hydrocarbons, Nat. Chem. 2023, 15, 377; DOI: https://www.nature.com/articles/s41557-022-01121-w published as preprint: ChemRXiv 2022, DOI: 10.26434/chemrxiv-2022-6pc2z This work was highlighted in "synfact of the month" in Synfacts 2023, 19, 0553. |
© 2021 Teichert group |
|
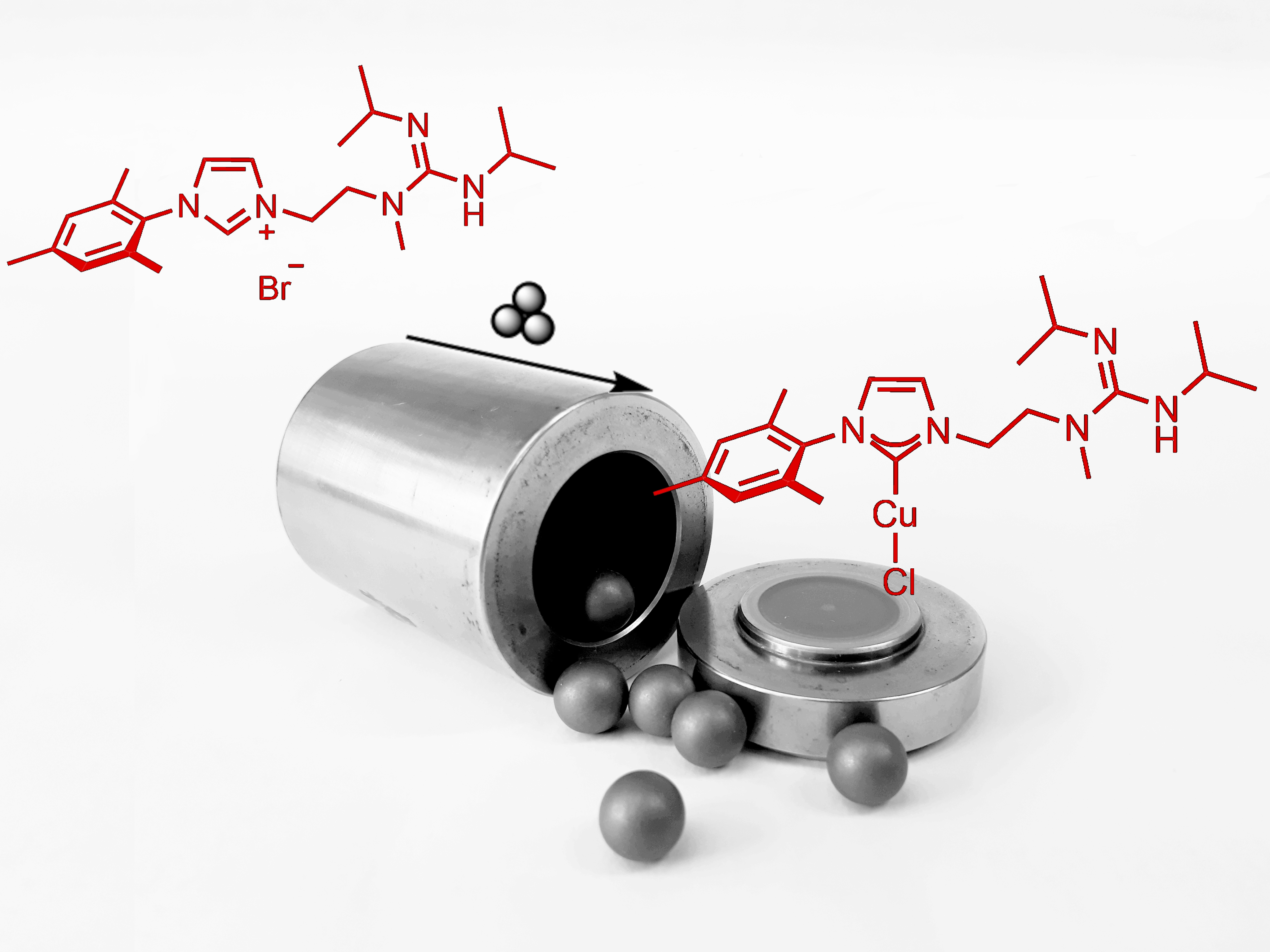
40. I. Remy-Speckmann, B.M. Zimmermann, M. Gorai, M. Lerch, J.F. Teichert, Mechanochemical solid state synthesis of a bifunctional copper(I)/N-heterocyclic carbene complex and its catalytic activity in hydrogenative transformations, Beilstein J. Org. Chem. 2023, 19, 440; DOI: https://doi.org/10.3762/bjoc.19.34
This work has been highlighted in ChemistryViews.
published as preprint: ChemRXiv 2021, DOI: 10.33774/chemrxiv-2021-z5720
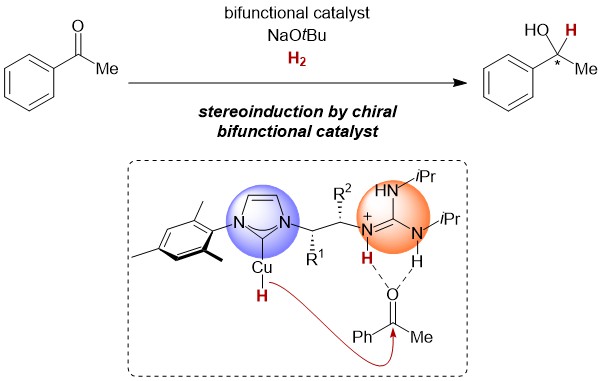
|
© 2021 Teichert group |
|
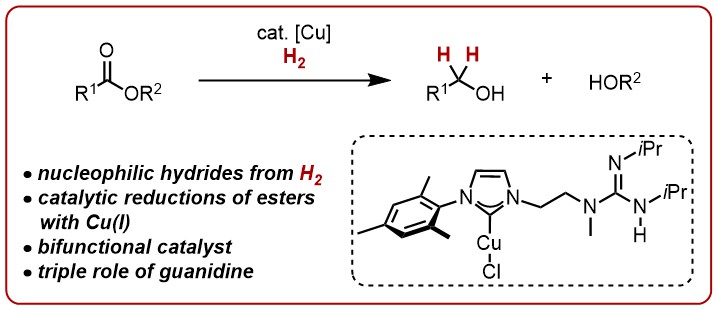
39. B.M. Zimmermann, T. Tran Ngoc, D.-I. Tzaras, T. Kaicharla, J.F. Teichert, A Bifunctional Copper Catalyst Enables Ester Reduction with H2: Expanding the Reactivity Space of Nucleophilic Copper Hydrides, J. Am. Chem. Soc. 2021, 143, 16865; DOI: https://doi.org/10.1021/jacs.1c09626
published as a preprint: ChemRXiv 2021, DOI: 10.26434/chemrxiv.14730987.v1
This work was highlighted in Org. Proc. Res. Dev. DOI: https://doi.org/10.1021/acs.oprd.1c00447 as item of interest.
This work was highlighted in Synfacts 2022, 18, 0050; DOI: 10.1055/s-0041-1737210
|
© 2021 American Chemical Society |
|
38. F. Czerny, K. Searles, P. Šot, J.F. Teichert, P.W. Menezes, C. Copéret, M. Driess, Well-Defined, Silica-Supported Homobimetallic Nickel Hydride Hydrogenation Catalyst, Inorg. Chem 2021, 60, 5483; DOI: https://doi.org/10.1021/acs.inorgchem.0c03188. |
© 2020 American Chemical Society |
|
37. M. König, M. Rigo, N. Chaoui, T. Tran Ngoc, J. D. Epping, J. Schmidt, P. Pachfule, J. F. Teichert, M. Drieß, A. Thomas, Immobilization of an Iridium Pincer Complex in a Microporous Polymer for Application in Room-Temperature Gas Phase Catalysis, Angew. Chem. Int. Ed. 2020, 45, 19830. DOI: 10.1002/anie.202004092. |
© 2020 Wiley-VCH |
|
36. L. T. Brechmann, J. F. Teichert, Catch It if You Can: Copper-catalyzed (Transfer) Hydrogenation Reactions and Coupling Reactions by Intercepting Reactive Intermediates thereof, Synthesis 2020, 52, 2483-2496. This work has been selected as "editor's choice" and is now open access. |
© 2020 Thieme |
| 35 .N. O. Thiel, F. Pape, J. F. Teichert, Homogeneous Hydrogenation with Copper Catalysts (Book Chapter) in Homogeneous Hydrogenation with Non-Precious Catalysts, Wiley, Weinheim, 2019. |
© 2019 Wiley-VCH |
|
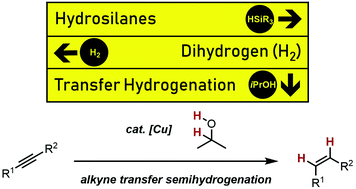
34. T. Kaicharla, B. M. Zimmermann, M. Oestreich, J. F. Teichert, Using alcohols as simple H2-equivalents for copper-catalysed transfer semihydrogenations of alkynes, Chem. Commun. 2019, 55, 13410-13413. This work was highlighted by ChemistryViews and by Organic Chemistry Highlights. |
© 2019 RSC |
|
33. N. O. Thiel, B. Kaewmee, T. Tran Ngoc, J. F. Teichert, A Simple Nickel Catalyst Enabling an E-Selective Alkyne Semihydrogenation, Chem. Eur. J. 2020, 26, 1597-1603. |
© 2019 Wiley-VCH |
|
32. N. O. Thiel, L. T. Brechmann, J. F. Teichert, Catalytic Hydrogenations with Cationic Heteroleptic Copper(I)/N-Heterocyclic Carbene Complexes, Synlett 2019, 30, 783-786. Published as part of the Special Section 10th EuCheMS Organic Division Young Investigator Workshop. |
© 2019 Thieme |
|
31. B. M. Zimmermann, S. C. K. Kobosil, J. F. Teichert, Catalytic hydrogenation of alpha,beta-unsaturated carboxylic acid derivatives using coper(I)/N-heterocyclic carbene complexes, Chem. Commun. 2019, 55, 2293-2296. |
© 2019 RSC |
|
30. F. Pape, L. T. Brechmann, J. F. Teichert, Catalytic Generation and Chemoselective Transfer of Nucleophilic Hydrides from Dihydrogen, Chem. Eur. J. 2019, 25, 985-988. This work was featured in Organic Chemistry Highlights. |
© 2018 Wiley-VCH |
|
29. M. Das, T. Kaicharla, J. F. Teichert, Stereoselective Alkyne Hydrohalogenation by Trapping of Transfer Hydrogenation Intermediates, Org. Lett. 2018, 20, 4926-4929. This work was highlighted in Synfacts 2018, 14, 1265 and in Organic Chemistry Highlights. |
© 2018 American Chemical Society |
|
28. J. F. Teichert, Synthese im Blickpunkt: Benzol dearomatisieren mit Licht, Nachr. Chem. 2017, 65, 1092-1095. |
© 2017 Wiley-VCH |
|
27. T. N. T. Nguyen, N. O. Thiel, J. F. Teichert, Copper(I)-catalysed asymmetric allylic reductions with hydrosilanes, Chem. Commun. 2017, 53, 11686-11689. |
© 2017 RSC |
|
26. F. Pape, J. F. Teichert, Synthese im Blickpunkt: Polyketide am Fließband, Nachr. Chem. 2017, 65, 879 - 883. |
© 2017 Wiley-VCH |
|
25. N. O. Thiel, J. F. Teichert, Synthese im Blickpunkt: Interhalogenierung: Das Problemkind der Alkenbromierung, Nachr. Chem. 2017, 65, 772 - 775. |
© 2017 Wiley-VCH |
|
24. N. O. Thiel, S. Kemper, J. F. Teichert, Copper(I)-catalyzed stereoselective hydrogenation of 1,3-diynes and enynes, Tetrahedron 2017, 73, 5023-5028. (Invited manuscript for the Symposium-in-Print on the occasion of the Tetrahedron Prize 2017 for Ben Feringa) |
© 2017 Elsevier |
|
23. J. F. Teichert, Synthese im Blickpunkt: Benachbarte Stereozentren unter voller Kontrolle, Nachr. Chem. 2017, 65, 530 - 534. |
© 2017 Wiley-VCH |
|
22. M. Trunk, J. F. Teichert, A. Thomas, Room-Temperature Activation of Hydrogen by Semi-Immobilized Frustrated Lewis Pairs in Microporous Polymer Networks, J. Am. Chem. Soc. 2017, 139, 3615-3618. |
© 2017 American Chemical Society |
|
21. F. Pape, J. F. Teichert, Dealing at Arm’s Length - Catalysis with N-Heterocyclic Carbene Ligandsbearing Anionic Tethers (Microreview), Eur. J. Org. Chem. 2017, 29, 4206-4229. |
© 2017 Wiley-VCH |
|
20. F. Pape, J. F. Teichert, Tethered NHC Ligands for Stereoselective Alkyne Semihydrogenations, Synthesis 2017, 49, 2470-2482. |
© 2017 Thieme |
|
19. J. F. Teichert, Synthese im Blickpunkt: C-C Bindungen knüpfen mit Aktivestern, Nachr. Chem. 2017, 65, 26-29. |
© 2017 Wiley-VCH |
|
18. E. Korytiaková, N. O. Thiel, F. Pape and J. F. Teichert, Copper(I)-Catalysed Transfer Hydrogenations with Ammonia Borane, Chem Commun. 2017, 53, 732-735. |
© 2016 RSC |
|
17. N. O. Thiel and J. F. Teichert, Stereoselective alkyne semihydrogenations with an air-stable copper(I) catalyst, Org. Biomol. Chem. 2016, 14, 10660-10666. This work was highlighted in Synfacts 2017, 13, 57 and in Organic Chemistry Highlights. |
© 2016 RSC |
|
16. T. N. T. Nguyen, N. O. Thiel, F. Pape and J. F. Teichert, Copper(I)-catalyzed Allylic Substitutions with a Hydride Nucleophile, Org. Lett. 2016, 18, 2455-2458. |
© 2016 American Chemical Society |
|
15. F. Pape, N. O. Thiel and J. F. Teichert, Z-Selective Copper(I)-catalyzed Alkyne Semihydrogenation with tethered Cu-alkoxide complexes, Chem. Eur. J. 2015, 21, 15934-15938. Highlighted in Nachr. Chem. 2016, 64, 279. |
© 2015 Wiley-VCH |
Publications from non-independent research
14. A. K. Schoonen, M. A. Fernández-Ibáñez, M. Fañanás-Mastral, J. F. Teichert, B. L. Feringa,Chiral amides via copper-catalysed enantioselective conjugate addition, Org. Biomol. Chem. 2014, 12, 36-41.This article was selected as an OBC hot article.
13. J. F. Teichert, D. Mazunin, J. W. Bode,Chemical Sensing of Polyols with Shapeshifting Boronic Acids as a Self-Contained Sensor Array, J. Am. Chem. Soc. 2013, 135, 11314 – 11321. This work was highlighted as a JACS Spotlight: J. Am. Chem. Soc. 2013, 135, 11679 and by Max von Delius in Nachrichten aus der Chemie 2014, 62, 522-525.
12. M. Fañanás-Mastral, J. F. Teichert, J. A. Fernández-Salas, D. Heijnen, B. L. Feringa, Enantioselective synthesis of Almorexant via Iridium-catalysed intramolecular allylic amidation, Org. Biomol. Chem. 2013, 11, 4521 – 4525.Highlighted in Synfacts 2013, 9, 921.
11. K. K. Larson, M. He, J. F. Teichert, A. Naganawa, J. W. Bode, Chemical Sensing with Shapeshifting Organic Molecules, Chem. Sci. 2012, 3, 1825-1828. This work was highlighted by Stuart Cantrill in Nature Chemistry: Nature Chem. 2012, 4, 336-337.
10. J. F. Teichert, M. Fañanás-Mastral, B. L. Feringa,Synthetic approaches to highly functional β-carboline building blocks via allylic amidation, Synthesis 2012, 44, 409-416.
9. A. L. Gottumukkala, J. F. Teichert, D. Heijnen, N. Eisink, S. van Dijk, C. Ferrer, A. van den Hogenband, A. J. Minnaard,Pd-Diimine: A Highly Selective Catalyst System for the Base-Free Oxidative Heck Reaction, J. Org. Chem. 2011, 76, 3498-3501. Highlighted in Synfacts 2011, 8, 894.
8. J. F. Teichert, T. den Hartog, M. Hanstein, C. Smit, B. ter Horst, V. Hernandez-Olmos, B. L. Feringa, A. J. Minnaard, Organocatalytic Reduction of Carbon-Carbon Double Bonds in Racemization-sensitive Compounds, ACS Catal. 2011, 1, 309-315.
7. J. F. Teichert, B. L. Feringa, Catalytic Asymmetric Conjugate Addition of Grignard Reagents to Coumarins – Synthesis of Versatile Chiral Building Blocks, Chem. Commun. 2011, 47, 2679-2681. Highlighted in Synfacts 2011, 6, 642.
6. J. F. Teichert, M. Fañanás-Mastral, B. L. Feringa, Ir-catalyzed asymmetric allylic amidation – Enantioselective Synthesis of Chiral Tetrahydroisoquinolines, Angew. Chem. Int. Ed. 2011, 50, 688-691; Angew. Chem. 2011, 123, 714-717.Highlighted in Synfacts 2011, 4, 419.
5. J. F. Teichert, S. Zhang, A. W. van Zijl, J. W. Slaa, A. J. Minnaard, B. L. Feringa, Cu-Catalyzed Asymmetric Allylic Alkylation in Combination with Ru-Catalyzed Metathesis: A Straightforward Approach to Chiral N-Heterocycles, Org. Lett. 2010, 12, 4658-4660. Highlighted in Synfacts 2010, 12, 1392.
4. J. F. Teichert, B. L. Feringa, Catalytic Asymmetric Synthesis of 2,5-Naphthylpyrrolidine, Synthesis 2010, 7, 1200-1204.
3. J. F. Teichert, B. L. Feringa, Phosphoramidites – Privileged Ligands in Asymmetric Catalysis, Angew. Chem. Int. Ed. 2010, 49, 2486-2528; Angew. Chem. 2010, 122, 2538-2582.
2. J. F. Teichert, P. Oulié, K. Jacob, L. Vendier, M. Etienne, R. M. Claramunt, C. López, C. P. Medina, I. Alkorta, J. Elguéro, The strucutre of the Replacement of an H by a F Atom on the Supramolecular Structure of NH-Indazoles, New J. Chem. 2007, 31, 936-946.
1. P. Oulié, J. F. Teichert, L. Vendier, C. Dablemont, M. Etienne, Aromatic interactions in hydrotris(3-methylindazolyl)-borate organoniobium complexes: control of an alkyne ligand orientation in the crystal, New J. Chem. 2006, 30, 679-682.