Mechanochromic materials for force sensing
Stress and strain are ubiquitous phenomena in polymeric materials.[1] During polymer synthesis, processing, analysis and usage, force of varying magnitude is – intentionally or unintentionally - applied to long chain molecules. Our group is developing concepts to make stress and strain visible at any time, which is achieved by molecular stress reporters that change color upon mechanical activation. These so-called mechanophores translate macroscopic and microscopic stress into different optical properties, which is monitored in-situ by steady-state UV-Vis and photoluminescence spectroscopy.Spiropyran-functionalized polymers
Spiropyran (SP) is a well-known molecule that undergoes reversible reaction to merocyanine (MC) upon various stimuli.[2] Upon appropriate attachment to polymers, this reaction can also be triggered by force.[3] We have been investigating how substituent effects influence this equilibrium, and how copolymerization of SP-based monomers furnish different polymers with interesting mechanical properties.[4–10]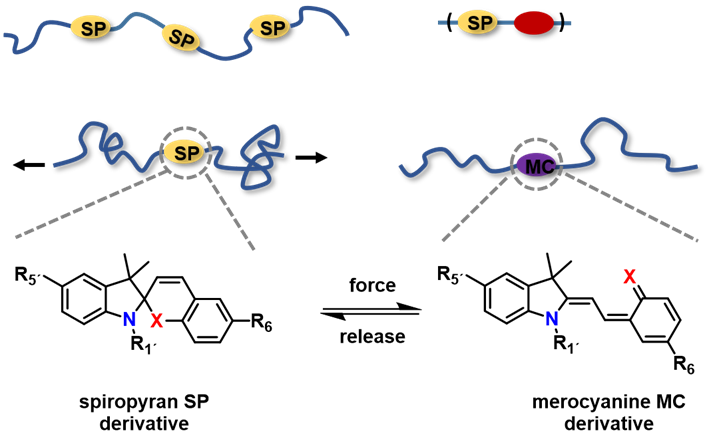
Transient mechanochromic behaviour
Transient mechanochromic behavior, i.e. a force-induced, non-permanent color change, can be generated when a phenyl ring is used as substituent at C-6. Synthetically, dibromospiropyran is copolymerized with phenylene-based comonomers under Suzuki conditions. In order to incorporate SP into a polyphenylene with suitable mechanical properties, we designed kinked polyphenylenes PmmpP with outstanding toughness and large strain at break. Plastic deformation of PmmpP with incorporated SP reveals that the stress-induced color change is only stable under applied stress, but instantaneously vanishes upon force release. The origin of this new behavior is an electronic effect of the phenyl unit at C-6, which makes MC rather unstable compared to SP, such that it can only form under applied stress.[11]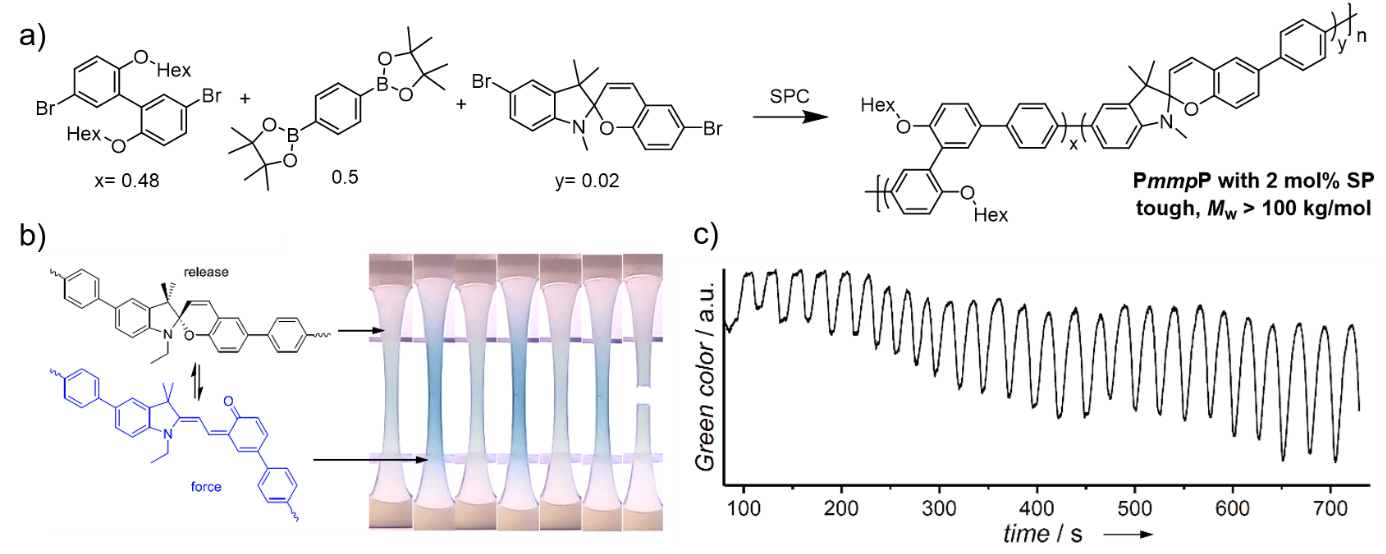
Anisotropic force sensors with reduced onset strain of color formation
In mechanochromic polymers, the onset of color formation occurs often at ~50 % strain as a result of chain orientation, preceding mechanical activation. This poses a strong limitation with respect to sensitivity of the force sensor and thus limits their applicability. We have used electrospinning to orient SP copolymer chains within fibers, which reduces the onset strain to below 10%. Embedding fibers into elastomeric PMDS allowed us to fabricate highly sensitive and anisotropic force sensors that additionally exhibit transient mechanochromic behavior.[12]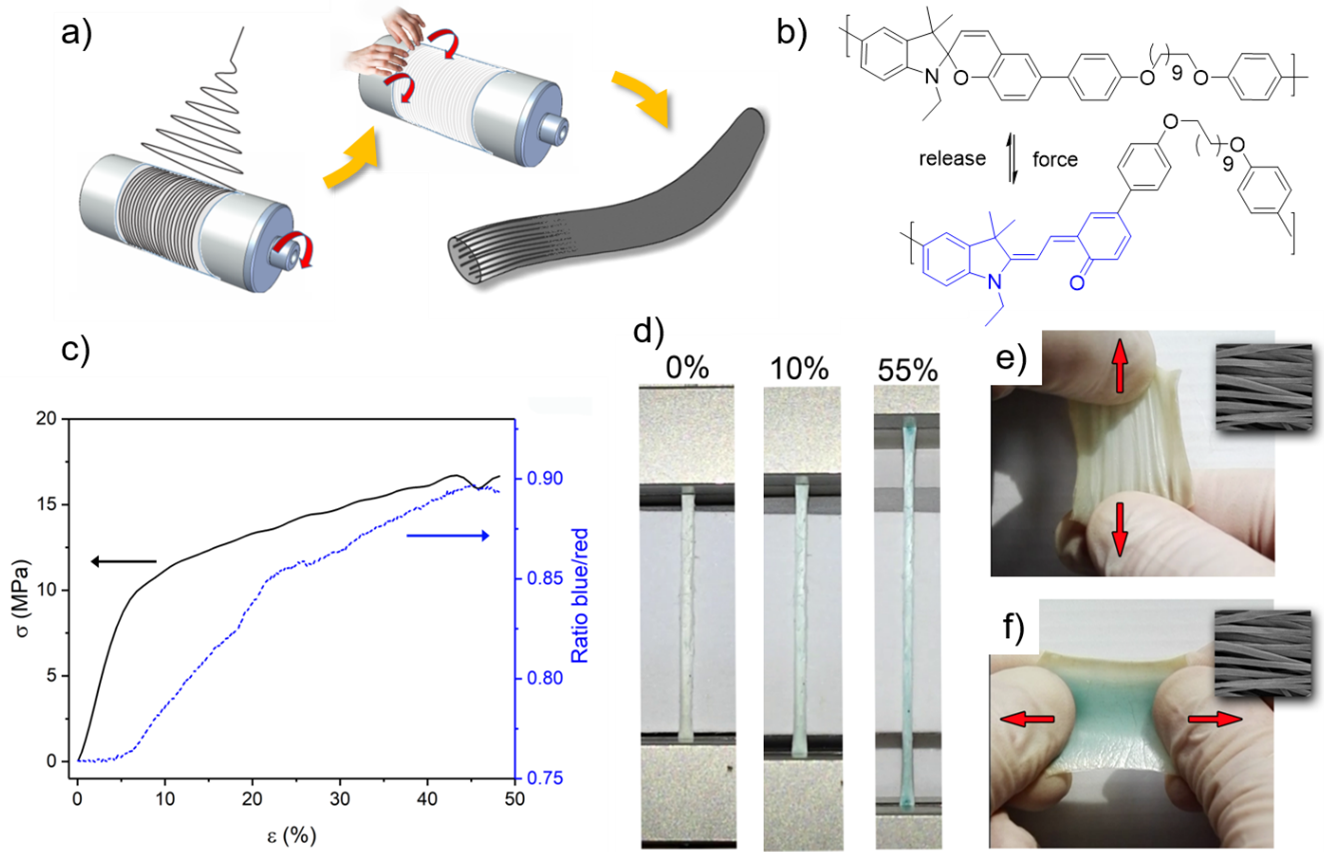
Force-responsive donor-acceptor torsional springs
A generic pitfall of the SP/MC system, and also of other mechanophores, is that they are two-state systems that are either switched of (colorless) or on (colored). Correlating forces of different magnitude with continuous changes in the optical properties, however, is much more challenging to realize. We have recently introduced the new concept of donor acceptor (DA) torsional springs, which are sterically encumbered chromophores with torsion-limited conjugation. By appropriate covalent attachment of polymer chains and the application of macroscopic stress, these DA springs can be mechanically forced out of their equilibrium geometry towards coplanarity. The resulting increased conjugation causes bathochromic shifts in absorption and photoluminescence spectra, a process that allows gradual changes to be monitored. Such deformation of the DA spring is reversible, with the design of the torsion potential of the spring allowing to tune the force range.[13] Further application and development of DA-springs allows for e.g. the determination of entanglement molecular weight of PmmpP[14], yielding values in excellent agreement with rheology data.[15]
Our current activities in the development of next generation ansa-DA springs aim at improving sensitivity[16], force calibration and the detection of residual stresses in polymeric materials.
Further application and development of DA-springs allows for e.g. the determination of entanglement molecular weight of PmmpP[14], yielding values in excellent agreement with rheology data.[15]
Our current activities in the development of next generation ansa-DA springs aim at improving sensitivity[16], force calibration and the detection of residual stresses in polymeric materials.
References
[1] M. M. Caruso et al., Chem. Rev - 2009.
[2] R. Klajn, Chem. Soc. Rev. - 2013.
[3] D. A. Davis et al., Nature - 2009.
[4] H. Komber et al., Polym. Chem. - 2013.
[5] M. Sommer, Macromol. Rapid Commun. - 2013.
[6] L. Metzler et al., Polym. Chem. - 2015.
[7] O. Brügner et al., J. Phys. Chem. A - 2017.
[8] S. B. Schmidt et al., Polym. Chem. - 2017.
[9] O. Brügner et al., Phys. Rev. Mater. - 2018.
[10] M. Sommer, Rapid Commun. - 2021.
[11] F. Kempe et al., Angew. Chem. Int. Ed. - 2018.
[12] M. Raisch et al., Adv. Electron. Mater. - 2018.
[13] M. Raisch et al., Nat. Commun. - 2021.
[14] M. Raisch et al., ACS Macro Lett. - 2022.
[15] A. M. Fenton et al., ACS Cent. Sci. - 2022.
[16] R. Hertel et al., J. Am. Chem. Soc. - 2022.
