|
POLYANILINE - CLAY NANOCOMPOSITES AS CORROSION PROTECTION Polyaniline is unique in the family of conjugated polymers [1] and the most intensively investigated electronically conductive polymer because of its potential commercial applications in e.g. rechargeable batteries, electrochromic display devices, electrochemical sensors, electrochemical capacitors and in the last few years in active corrosion protection [2, 3, 4, 5, 6, 7]. Recently, conducting polymer layered inorganic solid nanocomposites have been the subject of considerable research interest because, being derived from a unique combination of inorganic and organic components they have possible technological application as well as scientific issues concerning them[8, 9, 10] . One of the most prevalent class of these nanocomposites is composed of materials containing polyaniline (PANI) and montmorillonite (MMT) (clay minerals), because MMT minerals have attractive advantages such as large surface area, ion exchange and expandability properties. 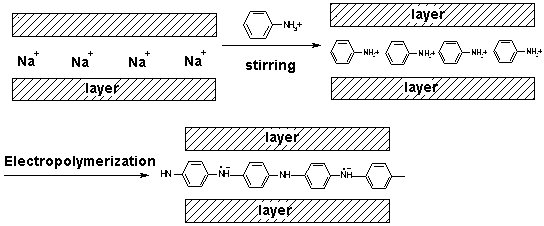 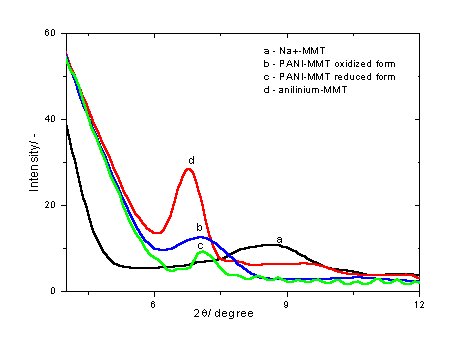 Referenzes: [1] Huang, J.; Kaner, R. B. J. Am. Chem. Soc. 2004, 126, 851. [2] Gasparac, R.; Martin, C. R. J. Electrochem. Soc. 2001, 148, B138. [3] do Nascimento, G. M.; Constantino, V. R. L; Landers, R.; Temperini, M. L. A. Macromolecules 2004, 37, 9373. [4] Kim, B. H.; Jung, J. H.; Hong, S. H.; Joo, J. Macromolecules 2002, 35, 1419. [5] Chen, K. H.; Yang, S. M. Synth. Met. 2003, 135-136, 151. [6] Kim, B. H.; Jung, J. H.; Kim, J. W.; Choi, J. H.; Joo, J. Synth. Met. 2001, 117, 115. [7] Conroy, K. G.; Breslin, C. B. Electrochim. Acta 2003, 48, 721. [8] Yoshimoto, S.; Ohashi, F.; Ohnishi, Y.; Nonami, T. Synth. Met. 2004, 145, 265. [9] Tyan, H. L.; Liu, Y. C.; Wei , K. H. Chem. Mater. 1999, 11, 1942. [10] Yeh, J. M.; Chen, C. L.; Chen, Y. C.; Ma , C. Y.; Lee, K. R.; Wei, Y.; Li, S. Polymer 2002, 43, 2729. [11] Lee, D.; Char, K.; Lee, S. W.; Park,Y. W. J. Mater. Chem. 2003, 13, 2942. [12] Wu, Q.; Xue, Z.; Qi, Z.; Wang, F. , Polymer 2000, 41, 2029. [13] Lee, D.; Lee, S. H.; Char, K.; Kim, J. Macromol. Rapid Commun. 2000, 21,1136. [14] de Azevedo, W. M.; Schwartz, M. O. E.; do Nascimento, G. C.; da Silva, E. F.; Phys. stat. sol. (c) 2004, 1, S249. [15] Kim, J. W.; Kim, S. G.; Choi, H. J.; Jhon, M. S. Macromol. Rapid Commun. 1999, 20, 450. [16] Genies, E. M.; Boyle, A.; Lapkowski, M.; Tsintavis, C. Synth. Met. 1990, 36, 139. [17] Inoue H.; Yoneyama, H. J.Electroanal. Chem. 1987, 233, 291. [18] Feng, B.; Su, Y.; Song, J.; Kong, K. J. Mater. Sci. Lett. 2001, 20, 293. |
