Spectroelectrochemistry of intrinsically conducting Furan-Thiophene copolymers
Introduction:
Electronically conducting polymers, in particular those based on monomers such as polypyrrole, polythiophene, polyselenophene and their derivatives have many potential applications, for example in: materials for battery electrodes [1], gas sensors [2], chemical sensors [3], ion sieving [4], corrosion protection [5] and microwave shielding [6].
Among polymer-conducting materials with an extended pi-electron system, polyfuran is interesting because of its possible technological applications as a humidity sensor [7]. Polyfuran is very sensitive to humidity, and its electrical resistivity decreases considerably and reversibly upon contact with moisture.
Polyfuran is among the most ill-defined of conjugated polymers compared to polypyrrole and polythiophene. This polymer was claimed to have been obtained previously by electrochemical polymerisation of furan, but the high voltage required for the electropolymerization (1.8 - 2.5 V) results in irreversible oxidation of the polymer [8].
The synthesis of polyfuran films was first mentioned by Touillon and Garnier, the reported polymer exhibited low electrical conductivity which ranged from 10-5 - 10-3 Scm-1 [9]. With another method, polyfuran was prepared by electrochemical reduction of 2,5-dibromofuran in acetonitrile using Ni(bipy)32+ as catalyst. The obtained polymer on the cathode was in its neutral insulating form and only in trace amount [10].
Gleins and Benz tried to use terfuran as the starting monomer to lower the oxidation potential. The obtained polymer has electrical conductivity of 2*10-3 Scm-1 when doped with CF3SO3-. Although the oxidation potential was lowered by this method (~1.5 V), the resultant polymer contained a large amount of saturated rings as evidenced by its Infra red spectrometry. [11].
More recently, furan was electropolymerized in acetonitrile at potentials between 2.1-2.3 V vs SCE using NaClO4 [12]. However the obtained film showed ill-defined cyclic voltammetry characterization compared with those obtained by Gleins and by Zotti. It was obvious that the polymer undergoes irreversible oxidation and contained a large amount of saturated rings or ring opened components under such high potentials.
Conductive polyfuran and poly(2-methylfuran) were prepared by controlled potential electrolysis in acetonitrile at room temperature. The electrical conductivity of polyfuran is decreased by a factor of 4 when the methyl group is present in the a position. The temperature effect on the rate of polymerisation of furan was studied by a cyclic voltammetry technique and the mechanism of electrochemical polymerisation was investigated by in situ electron spin resonance [13].
Free-standing polyfuran films have been successfully synthesized by electrochemical polymerisation of furan at lower potential (1.2 vs Ag/AgCl ) in a binary solvent system consisting of boron trifluoride-ethyl ether and additional amount ethyl ether. The polymer shows good mechanical properties, its electrical conductivity around 10-2 Scm-1 [14].
Furan and pyrrole were electrochemically copolymerised in a binary solvent system consisting of boron trifluoride ethyl ether and additional ethyl ether. The influence of applied polymerisation potential and the monomer feed ratio of furan and pyrrole on the copolymers were investigated. The film obtained was characterized by cyclic voltammetry, infra red and raman spectroscopies [15].
Electrochemical copolymerisation of furan and 3-methyl thiophene was performed potentiostatically in a binary solvent system consisting of boron trifluoride-ethyl ether and additional amount ethyl ether (ratio 1:2). The obtained copolymer was characterized by cyclic voltammetry, Infrared spectroscopy and electrical conductivity. The copolymerisation is carried out at 1.2 V vs Ag/AgCl/ 0.1 M KCl, the polymer has an electrical conductivity of 0.36 Scm-1 [16].
Aim of the proposed research:
Due to the lower aromaticity and drastic conditions needed for polymerisation of furan, it is difficult to electrooxidize and electropolymerize and a number of saturated rings in the polymer chains is obtained. Hence, less attention has been focused on the study of the properties and potential applications of polyfuran.
In the present proposal, we aim to prepare furan-thiophene copolymers by electrochemical copolymerisation of furan and thiophene potentiostatically in a binary solvent system consists of boron trifluoride-ethyl ether and additional amount ethyl ether ( 1:2 ).
The copolymers will be studied by using spectroelectrochemical techniques beyond cyclic voltammetry, in particular in situ UV-Vis spectroscopy, in situ electrical conductivity measurements, in situ Infrared spectroscopy and finally in situ Raman spectroscopy.
The influence of monomer ratios and polymerisation potentials on the copolymers will be also investigated.
As a result, a deeper understanding of the spectroelectrochemical properties of the furan-thiophene copolymers which might be considered more promising for technological applications.
Experimental
- Materials:
Furan (Aldrich, 99%) and thiophene (Fluka, 98%) will be distilled under nitrogen just prior to use. Ethyl ether (EE) (Acros) will be dried and distilled in the presence of sodium. Boron trifluoride-ethyl ether (BFEE) (Acros, 48% BF3) will be used as received. Tetrabutylammonium tetrafluoroborate (TBATFB) (Aldrich, 99%) will be dried under vacuum at 80oC for 24 hours. Acetonitrile (Merck, anhydrous, max. 10 ppm H2O) will be used as received.
- Electrochemical experiments:
Electrochemical studies and copolymerisation of furan and thiophene will be performed potentiostatically in BFEE + EE (ratio 1:2) solution within a one-compartment three-electrode cell at room temperature. Platinum sheet electrode will be used as working electrode, Ag/AgCl electrode will be used as reference electrode. TBATFB will be used as supporting electrolyte in acetonotrile as solvent. Cyclic voltammetry experiments will be carried out in a monomer free acetonitrile solution containing TBATFB as supporting electrolyte. Before each experiment the solution will be deareated by the bubbling of N2.
- Spectroscopy experiments:
Optically transparent indium oxide (ITO) coated glass electrode will be used for measuring in situ UV-Vis spectra of the deposited polyfuran, polythiophene and copolymers using a UV-Vis scanning spectrophotometer, UV-2101 PC, Shimadzu. In situ Fourier transform infrared spectra will be recorded using BIO-RAD FTS-40 spectrophotometer at different potentials. In situ Raman spectra will be measured with a JOBIN YVON, T 64000.
- Measurements of electrical properties:
Conductivity measurements will be carried out using a band-gap gold electrode for both polyfuran, polythiophene and the copolymers.
-Preliminary results:
Some of preliminary results of this work are shown in the following figures:

Figure 1. The current-potential curves of (A) 0.10 M furan, (B) 0.10 M furan
and 0.10 M thiophene, (C) 0.10 M thiophene in BFEE + EE (ratio 1:2).
Scan rate: 100 mV/s. Reference electrode: Ag/AgCl.
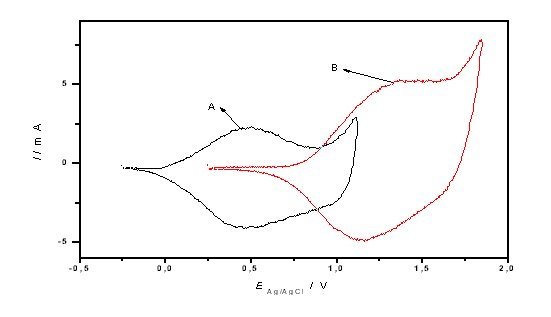
Figure 2. Cyclic voltammograms recorded in acetonitrile + 0.10 M TBATFB solution of (A) polyfuran, (B) polythiophene.
Scan rate: 100 mV/s. Reference electrode: Ag/AgCl.

Figure 3. Cyclic voltammograms recorded in acetonitrile+ 0.10 M TBATFB solution of
(A) polyfuran, (B) copolymer prepared at 1.60 V from BFEE + EE (ratio 1:2)
solution containing 0.10 M furan and 0.10 M thiophene,
(C) polythiophene. Scan rate: 100 mV/s. Reference electrode: Ag/AgCl.
REFERENCES
| 1. | J. Amanokura, Y. Suzuki, S. Imabayashi and M. Watanabe, J. Electrochem. Soc., 148 (2001) D43. |
| 2. | G. A. Stozing, S. M. Briglin, R. H. Grubbs and N. S. Lewis, Anal. Chem., 72 (2000) 3181. |
| 3. | C. W. Lin, B. J. Hwang and C. R. Lee, J. Appl. Polym. Sci., 73 (1999) 2079. |
| 4. | H. Shinaohara, M. Aizawa and H. Shirakawa, J. Chem. Soc. Chem. Commun., (1986) 87. |
| 5. | H. N. T. LE, B. Garcia, C. Deslouis and X. Q. LE, J. Appl. Electrochem., 32 (2002) 105. |
| 6. | L. J. Buckley and M. Eashov, Synth. Met., 78 (1996) 1. |
| 7. | E. Blanca, I. Carrillo, M. J. Gonzalez-Tejera and I. Hernandez-Fuentes, J Polym. Sci. : part A: polymer chemistry, 38 (2000) 291. |
| 8. | B. Nessakh, Z. Kotkowska-Machnik and F. Tedjar, J. Electroanal. Chem., 269 (1990) 263. |
| 9. | G. Tourillon and F.Garnier, J. Electroanal. Chem., 135 (1982) 173. |
| 10. | G. Zotti, G. Schiavon and N. Comisso, Synthetic Metals, 36 (1990) 337. |
| 11. | S. Glenis, M. Benz, E. LeGoff, J. L. Schindler, C. R. Kannewurt and M. G. Kanatzidis, J. Am. Chem. Soc., 115 (1993) 12519. |
| 12. | M. J. Gonzalez-Tejera, I. Carrillo and I. Hernandez-Fuentes, Synthetic Metals, 73 (1995) 135. |
| 13. | B. Demirboga and A. M. Onal, Synthetic Metals, 99 (1999) 237. |
| 14. | X. Wan, F. Yan, S. Jin, X. Liu and G. Xue, Chem. Mater., 11 (1999) 2400. |
| 15. | X. Wan, W. Zhang, S. Jin, G. Xue, Q. You and B. Che, J. Electroanal. Chem., 470 (1999) 23. |
| 16. | L. Li, W. Chen, N. Xu, Z. G. Xaio and G. Xue, J. Materials Science, 39 (2004) 2395. |
