Electrochemical Preparation and Spectroelectrochemical Characterization of Aniline-o-Aminophenol Copolymers
Intrinsically conducting polymers attract significant interest as promising candidates for various applications as electrocatalysts [1 - 4], electrochromic devices [5, 6], solar cells [7] and rechargeable batteries [8, 9]. Among conducting polymers, polyaniline has been studied extensively. However, the conductivity, the energy density, the catalytic activity, the electrochromic property and redox activity of polyaniline are strongly affected by pH values, which limit its application to a certain extent. This is mostly due to the fact, that polyaniline has low conductivity and little electrochemical activity at pH > 4 and its usable potential range decreases with increase in pH value. A possible way for solving this problem is to improve the properties of polyaniline, either via the polymerization of substituted monomers and post-treatment [10, 11] or copolymerization of aniline with other monomers. Yue et al. reported the successful preparation of sulfonic acid ring-substituted polyaniline by the reaction of the emeraldine base form of polyaniline with fuming sulfuric acid [12, 13]. This polymer has a conductivity of 0.1 S/cm, which is independent of pH in the aqueous solutions of pH = 7. Thus, the synthesis of self-doped polyaniline is a promising way to reduce the pH dependence of the conductivity of the polyaniline at pH > 4.
The copolymerization of aniline with N-methylaniline, N-butylaniline and 2-(4-aminophenyl)-6-methylbenzothiozole has been carried out using chemical methods [14 - 16]. Lukachova et al. reported the chemical copolymerization of aniline with m-aminobenzenesulfonic acid using ammonium persulfate as the oxidant [17].The copolymer prepared in this manner improves the stability upon cycling in neutral and alkaline media. Pioneering work for the electrochemical copolymerization of aniline with o- or m-toluidine was done by Wie et al. [18]. They reported that the conductivity of the copolymer can be controlled in a broad range depending on the monomer concentration ratio. Karyakin and coworkers [19, 20] reported on self doped polyaniline obtained from the electrochemical copolymerization of aniline with three substituted aniline, i.e. m-aminobenzoic acid, anthranilic acid and m-aminobenzenesulfonic acid.
The copolymer thus obtained can retain the redox activity in a buffer solution of pH 9 at the scan rate of 25 mV/s. It is clear, that the pH dependence of the electrochemical activity of the copolymer was improved pronouncedly, compared with the parent aniline. poly(aniline-co-m-aminobenzenesulfonic acid) prepared electrochemically has been used to fabricate the secondary Zn-copolymer battery,which has a rather high specific energy [21]. The electrochemical copolymerization of aniline and p-aminophenol has been carried out in the organic electrolyte [22]. This copolymer has potentiometric sensor function for phenol in aqueous solution. Recently ,Shaolin Mu reported the electrochemical copolymerization of aniline and o-aminophenol [23]. This preliminary study is only limited to the preparation and electrochemical activity of the copolymer. A lot of work is waiting to do with this polymer such as the effect of preparation conditions, copolymerization mechanism, electrochromic properties, applications and especially its structure.
PLANNED RESEARCH
In the present study aniline will be electrochemically copolymerized with orthoaminophenol in aqueous acidic solution. The effect of momomer concentration ratio on the copolymerization rate and the properties of copolymers will be investigated. The pH dependence of the copolymers will be studied in sodium sulfate solution of different pH values. The electrochemical properties of the copolymers will be discussed, on the basis of cyclic voltammetry and in situ conductivity measurements, and compared with those of homopolymers. In the next stage the copolymer will be subjected to the spectroelectrochemical investigation by using in situ UV-vis and in situ surface resonance Raman (SSR) spectroscopy. The spectroscopic properties of the copolymers will be compared with those of homopolymers. It is also intended to study the copolymer based on FT-IR spectrum and elemental analysis. Some preliminary results of this work are shown in the following figures:
 |
 |
 |
Figures
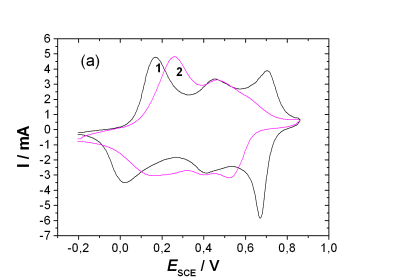
(a) Cyclic Voltammograms of (1) polyaniline and (2) Copolymer in 0.5 M H2SO4 solution at a scan rate of 50 mV/s
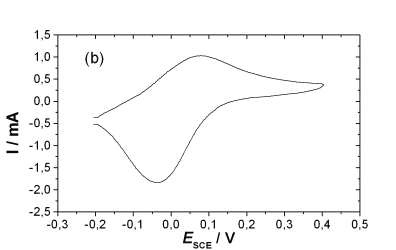
(b) Cyclic Voltammogram of polyorthoaminophenol in 0.5 M H2SO4 solution at a scan rate of 50 mV/s
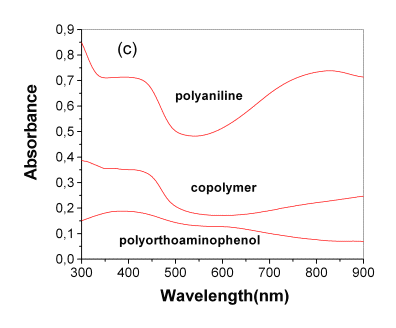
(c) in situ UV-Visible spectra taken in 0.5 M H2SO4 solution at 0.3V
REFERENCES
| 1. | Handbook of conducting polymers, vol. 1 and 2, Marcel Dekker, N.Y. (1986). |
| 2. | J.L. Bredas and G.B. Street, Acc.Chem.Res. 18 (1985) 309. |
| 3. | R. Noufi, A.J. Nozik, and J.F. Warren, J.Electrochem.Soc. 129 (1982) 2261. |
| 4. | G. Bidan, E.M. Genies, and M. Lapkowski, J. Chem. Soc. Chem. Commun.8(1998)533. |
| 5. | T. Kobayashi, N. Yoneyama, and H. Tamura, J. Electroanal. Chem. 177 (1984) 281. |
| 6. | C.D. Batich, H.A. Laitenen, and H.C. Zhou, J. Electrochem. Soc. 137 (1990) 883. |
| 7. | Y.H. Dong and S.L. Mu, Electrochim. Acta 36 (1991) 2015. |
| 8. | A.G. MacDiarmid, S.L. Mu, N.L.D. Somasiri, and W. Wu, Mol .Cryst. Liq. Cryst. 121 (1985) 187. |
| 9. | N. Oyama, T. Tatsuma, T. Sato, and T. Sotomura, Nature 373 (1995) 598. |
| 10. | R. Holze in:Handbook of Advanced Electronic and Photonic Materials and Devices Vol. 8 (H.S. Nalwa Ed.), Academic Press, San Diego 2001, p. 209. |
| 11. | R. Holze in: Advanced Functional Molecules and Polymers Vol. 2 (H.S. Nalwa Ed.), Gordon&Breach, Amsterdam 2001, p.171 |
| 12. | J. Yue and A.J. Epstein, J. Am. Chem. Soc. 112 (1990) 2800. |
| 13. | J. Yue, Z.H. Wang, K.R. Cromack, and A.J. Epstein, J. Am. Chem. Soc. 113 (1991) 2665. |
| 14. | J.J. Langer, Synth. Met. 35 (1990) 295. |
| 15. | J.Y. Bergeron and L.H. Dao, Polym. Commun. 32 (1991) 403. |
| 16. | M. Abdelazzem, S.H. Elhamouly, and A.A. Hathoot, Eur. Polym. J. 31 (1995) 1207. |
| 17. | L.V. Lukachova, E.A. Shkerin, E.A. Punganova, and E.E. Karyakin, J. Electroanal. Chem. 371 (1994) 259. |
| 18. | Y. Wei, R. Hariharan, and S.A. Patel, Macromolecules 23 (1990) 758. |
| 19. | A.A. Karyakin, A.K. Strakhova, and A.K. Yatsimirsdsky, J. Electroanal. Chem. 371 (1994) 259. |
| 20. | A.A. Karyakin, I.A. Maltsev, and L.V. Lukachova, J. Electroanal. Chem. 402 (1996) 217. |
| 21. | M.S. Rahmanifar, M.F. Mousavi, and M. Shamsipur, J. Power Sources 110 (2002) 229. |
| 22. | I.A. Vinokurov, S.Ya. Khaikin, V.V. Bertsev, and T.M. Karkhu, Elektrokhimiya 28 (1992) 181. |
| 23. | M. Shaolin, Synth. Met. 143 (2004) 259 |
