Anode materials with nanostructures for advanced lithium ion batteries
Introduction:
Li-ion battery technology is the best performing one owing to its delivered energy density (210Whokg-1; 650Whol-1), which exceeds any competing technologies by at least a factor of 2.51. So the lithium-ion batteries (LIBs) are widely applied in computers, mobile phones and other electronic equipment and even in hybrid power automobile. Exploring new high-capacity electrode materials is the key to develop the next-generation LIBs.
In order to overcome this problem, a new approach has been developed, namely self-assembly method, where the molecules are chemically adsorbed by making covalent bonds with the atoms on the substrate and form well defined structures [1]. SAMs offers a number of applications in sensors [2], catalysis [3], molecular electronic devices [4], and other areas [5]. SAMs have been formed from a variety of compounds and substrates including fatty acids, trichlorosilanes, trialkoxysilanes on glass, silicon, carboxylic acids, and alkyl phosphates on metal oxides [6]. So far most of the fundamental studies have been performed with aliphatic thiols and only very recently aromatic thiols moved into the focus of interest due to their highly anisotropic nature and high electrical conductivity. For anode materials of LIBs, commercial product is graphite, but its theoretical capacity is around 372 mAh/g, much lower than alloy anode material such as Si (4200 mAh/g), Sn (991 mAh/g) etc, also lower than transition metal oxides (MxOy, M = Sn, Fe, Co), such as SnO2 (781 mAh/g), Co3O4 (890 mAh/g), Fe3O4 (928 mAh/g)[2] etc. However, whether the alloy anode materials or the transition metal oxides both face the same challenge that they suffer from a large volume expansion and contraction during the discharge-charge cycling (shape change) which results in poor electrochemical behavior of the electrode, including rapid fading of the capacity and poor cycling lifetime (capacity fading).
Nanostructured materials and hybrid materials has been regarded as the most effective way to solve these above problems[3]. Metal oxides with diverse nanostructures (such as nanorods[4], nanowires[5], nanosheets[6], nanotubes[7], nanocages, spheres and hollow spheres[8], core and shell structure[9] etc.) were synthesized by hydrolysis and hydrothermal reaction, chemical vapor deposition, hard and soft templates methods etc. These special structured materials had been proved to possess greater properties than regular materials.
PLANNED RESEARCH:
Synthesize nanostructured metal oxides, such as SnO2 nanowires, nanorods, nano-hollow spheres, core-shell structured materials etc. by the hydrothermal reactions or template methods. Metal oxides composites with graphene or conductive polymers would be also very potential materials for Lithium ion batteries, for they can supply the good path for lithium ions and the electron which can confirm that these materials possess perfect rate capacity.
Explore their electrochemical properties (their performance as anode material for LIBs) and the reaction mechanism (so called conversion reaction when lithium reacts with metal oxide and the ion transfer process in porous electrode) by electrochemical methods (CV, EIS etc.)
|
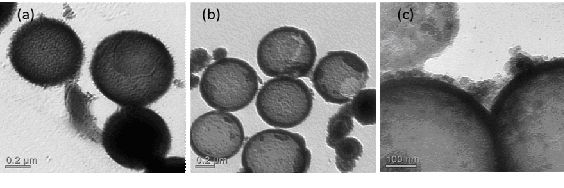
Fig 1, TEM of (a) SnO2/SiO2 composite spheres (b) SnO2 hollow spheres (c) PANi/SnO2 hollow spheres |
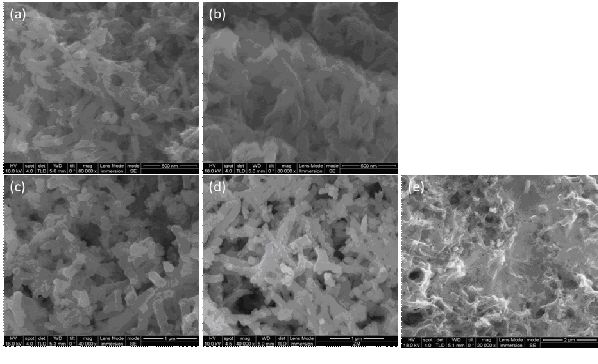 |
Fig 2,SEM of (a) PANi-1,(b)PANi-2,(c)PANi/SnO2-1, (d) PANi/SnO2-2(e) PANi/SnO2-3
REFERENCES
[1] Tarascon, J. M.; Armand, M., Issues and challenges facing rechargeable lithium batteries. Nature 2001,414 (6861), 359-367.
[2] Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J. M., Searching for new anode materials for the Li-ion technology: time to deviate from the usual path. Journal of power sources 2001,97-8, 235-239.
[3] (a) Arico, A. S.; Bruce, P.; Scrosati, B.; Tarascon, J. M.; Van Schalkwijk, W., Nanostructured materials for advanced energy conversion and storage devices. Nature Materials 2005, 4(5), 366-377; (b) Bruce, P. G.; Scrosati, B.; Tarascon, J.-M., Nanomaterials for rechargeable lithium batteries. Angewandte Chemie-International Edition 2008, 47 (16), 2930-2946.
[4] Vayssieres, L.; Graetzel, M., Highly Ordered SnO2 Nanorod Arrays from Controlled Aqueous Growth. Angewandte Chemie International Edition 2004, 43 (28), 3666-3670.
[5] Wang, Y.; Jiang, X.; Xia, Y., A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. Journal of the American Chemical Society 2003, 125 (52), 16176-16177.
[6] Sakaushi, K.; Oaki, Y.; Uchiyama, H.; Hosono, E.; Zhou, H.; Imai, H., Synthesis and Applications of SnO Nanosheets: Parallel Control of Oxidation State and Nanostructure Through an Aqueous Solution Route. Small 2010, 6 (6), 776-781.
[7] Ye, J.; Zhang, H.; Yang, R.; Li, X.; Qi, L., Morphology-Controlled Synthesis of SnO(2) Nanotubes by Using 1D Silica Mesostructures as Sacrificial Templates and Their Applications in Lithium-Ion Batteries. Small 2010, 6 (2), 296-306.
[8] Wang, B.; Chen, J. S.; Wu, H. B.; Wang, Z.; Lou, X. W., Quasiemulsion-Templated Formation of α-Fe2O3 Hollow Spheres with Enhanced Lithium Storage Properties. Journal of the American Chemical Society 2011.
[9] Lou, X. W.; Li, C. M.; Archer, L. A., Designed Synthesis of coaxial SnO @ carbon hollow nanospheres for highly reversible lithium storage. Adv. Mater 2009, 21, 2536-2539.