Spectroelectrochemistry Of Substituted Anilines
| Introduction
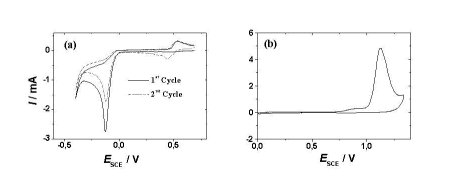
During the last few years much attention has been paid to investigations of monosubstituted benzenes and substituted anilines by spectroelectrochemical techniques [1, 2, 3]. Also this family of compounds was studied at different surfaces by cyclic voltammetry [4, 5]. In this study, we investigate the electrochemical behavior of nitroanilines, respective diamino compounds, and a new group of polymers (Polyvinylamine and Polyvinylamine functionalized with nitroanilines) by cyclic voltammetry at gold and platinum electrodes in acidic (0.1M HClO4) and neutral (0.1M KClO4) electrolyte solutions. In most cases electrochemical methods (e.g cyclic voltammetry, polarography, chronoamperometry) are coupled with spectroscopic methods to get more information about the adsorption orientation according to the electrode surface. Some work in this field was done for p-nitroaniline at platinum electrode using Modulation Reflectance Spectroscopy (MRS) [6] and for monosubstituted benzenes at gold electrode using Surface-Enhanced Raman Spectroscopy (SERS) [7]. SERS technique has attracted great attention in a variety of research field such as surface science, analytical chemistry, and nanotechnology because SERS allows one to measure Raman spectra from a highly dilute solution and from an ultra-thin film [8, 9]. Also in this study, the substituted anilines are subjected to SERS study on gold electrode in neutral and acidic perchlorate electrolyte solutions and at different electrode potentials. Moreover additional techniques like UV-Vis spectroscopy, Electron Spin Resonance spectroscopy (ESR) and quantum chemical calculation are included in planed studies. The figure below shows cyclic voltammogram samples of our result. |
||
 |
||
|
Cyclic voltammogram of a gold electrode in 0,1 M HClO4 saturated with |
||
|
References:
|
