NANOCRYSTALINE FLUOROPHOSPHATE CATHODES FOR ENHANCED VOLTAGE, RATE, LONG TERM CYCLING OF LITHIUM ION BATTERIES FOR EV and PHEV
Fluorophosphates like LiVPO4F showed high operating voltage (4.2V), long term cycle life (1260 cycles) and low capacity fade behavior compared to LiCoO2 (4.0V), LiFePO4 (3.5V), and other favorites such as LiFePO4F or LiFeSO4F, or NaFeSO4F, lead to the belief that it could replace the current cathode technology to enable the commercial production of low cost, high energy density and safe Li -ion batteries for EV and PHEV applications. However, electrolyte oxidation at higher potential and the formation of poly-vanadate compounds like Li3V2(PO4)3 as an impurity during the two step synthesis process must be controlled. By adopting appropriate surface modification, preferably by various ceramic oxides, carbon and phosphate (FePO4 and AlPO4 etc.,) based coatings would be employed to reduce activity towards electrolyte.
Soft-chemical syntheses offer numerous advantages for the formation of novel or advanced material forms including control over morphology (i.e., nanomaterials, coatings), phase, and composition. The controlled synthesis of nanomaterials and coatings, in particular, are imperative for the development of optical and electrical devices with ever-shrinking dimensions.
Substituting environmental friendly transition metal cations such as Co or Mn or Fe or Mg in between vanadium site using soft-chemical or precipitation methods, towards reducing the toxicity and the cost. We can synthesis LiVxFeyPO4F, LiVxMnyPO4F, and LiVxMgyPO4F by choosing LiOH.H2O, V2O5 in H2O2 or sodium vanadate or ammonium vanadate, Fe(NO3)xH2O,(NH4)2HPO4, and LiF or NH4F, and graphene. Thus, we propose to develop new and novel layer/3D-structures as 4V-cathodes other than LiVPO4F with at least a capacity of 100-160 mAh/g, and stable up to at least 800 cycles at 0.1C-1C-rate at ambient temperature and at 55°C.
The synthesis effort in this project were closely coupled with the characterization of electronic and geometric structure, surface and interfacial morphology by Rieveld refined XRD, DSC, TGA, XPS, solid state NMR, HREM, STM and neutron diffraction techniques. CV, GITT, and EIS studies of the cell at various temperatures and as a function of time will be performed. The capacity and cycle life of the synthesized electrode materials were measured via construction of the self designed cells.
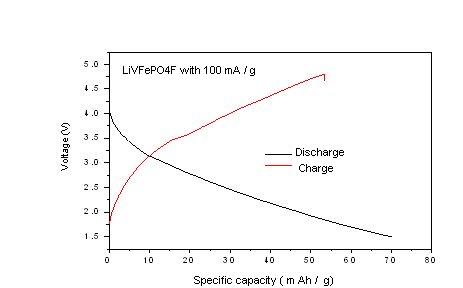
Fig 1, Charge- discharge profiles of LiVFePO4F cathode
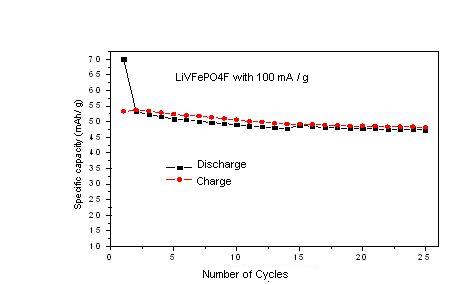
Fig 2, Charge-discharge cycle performance of LiVFePO4F cathode
The measured first discharge specific capacity (Fig. 1) of the LiVFePO4F cathode was 72 mAh/g in the potential range 5 to 1.5 V at current density 100mA/g. Fig. 2 shows 25 complete charge-discharge cycles of the battery that uses LiVFePO4F as a cathode. The measured specific discharge capacity was 53 mAh/g after 25 cycles of the LiVFePO4F. The cell exhibits 25 % capacity loss, which shows moderate cycle stability.
