




IV. LECTURE COURSES
A. Introduction
The table below shows all the required courses of the "Grundstudium" and "Hauptstudium", the number
of semesters that each requires, and when they are to be taken. The table also lists the ECTS credit
points assigned to each course. It should be noted that the typical ECTS student will only be enrolled
in courses of the Hauptstudium; however, if Grundstudium courses are desired or necessary, there is no
difficulty in assigning ECTS credits to them.
Table of lessons, physics curriculum for Master's Dregree
| Semester | Experimental
Physics | Theoretical
Physics | Higher
Mathematics
| Subsidiary
Subjects | Practicum
(Lab Courses)
| Hours
per
Week
|
| 1 (30 cr)
| I Mechanics,
Thermo-
dynamics
(11 cr) 4/2/2*
|
| Analysis I
Algebra I
(12 cr) 6/3/0
| Computer Science I
(5 cr) 2/2/0
| Practicum
in Physics I
(2 cr) 2
| 21 + 2*
|
| 2 (30 cr)
| II Elektro-
megnetism,
Optics
(11 cr) 4/2/2*
|
| Analysis II
Algebra II
(12 cr) 6/3/0
|
| Practicum
in Physics II
(7 cr) 4
| 19 + 2*
|
| 3 (30 cr)
| III Atomic and
Molecular
Physics
(8 cr) 4/2/0
| I Mechanics
(8 cr) 4/2/0
| Analysis III
(7 cr) 4/2/0
| Chemistry I
(2 cr) 2/0/0
| Practicum
in Physics III
(5 cr) 4
| 24
|
| 4 (30 cr)
| IV Nuclei and
Elementary
Particles
(5 cr) 2/2/0
| II Quantum
Mechanics
(8 cr) 4/2/0
| Analysis IV
(7 cr) 4/2/0
| A:
Chemistry II
(2 cr) 2/0/0B:
Coputer Science II
(5 cr) 2/2/0
| Practicum
in Physics IV
(2 cr) 2Practicum
in Chemistry
A: 4 / B: 2
(6 cr / 3 cr)
| 24
|
| First Examination
|
| 5 (30 cr)
| Physics of
Condensed
Mater II
(7 cr) 3/1/0
| III Electro-
dynamics
(11 cr) 4/2/0
|
| 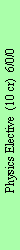
| 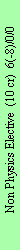
| Advanced
Practicum
I
(6 cr) 6
| 20
|
| 6 (30 cr)
| Physics of
Condensed
Mater II
(7 cr) 2/1/0
| IV Statistics,
Thermo-
dynamics
(11 cr) 4/2/0
|
| Advanced
Prakticum
II
(6 cr) 6
| 20
|
| 7 (30 cr)
| Atomes,
Molecules,
Lasers
(5 cr) 2/1/0
| V Continuum
Physics
(11 cr) 4/2/0
|
| Laboratory
Prakticum
I
Overview
(8 cr) 6
| 18
|
| 8 (30 cr)
| Selected
Topics from
Modern Physics
(4 cr) 2/0/0
| VI Selected
Topics from
Theoretical
Physics
(8 cr) 4/0/0
| Advanced
Seminar
(4 cr) 0/2/0
| Laboratory
Practikum II
Specialization
(12 cr) 6
| 14
|
| Final Examination
|
9
10
| Diplom Thesis
(60 cr)
|
+/o/* : + = Lecture / o = Discussion section / * = group learning (tutorial) in place of required seminar course
A: Chemistry elective / B: Computer Science elective
( _ cr): ECTS credit points
|
Distribution of semester credits:
| Grundstudium (4 semesters) | | 120 cr
|
| Hauptstudium (6 semester2) | | 180 cr
|
| davon:
|
| – experimenta physics | 23 cr |
|
| – theoretical physics | 41 cr |
|
| – advanced seminar | 4 cr |
|
| – requirde elective in physics | 10 cr |
|
| – requirde elective in another area | 10 cr |
|
| – advanced laboratory practical | 12 cr |
|
| – laboratory practical | 20 cr |
|
| – diplom thesis | 60 cr |
|
| Total | | 300 cr
|
Note:
The ECTS credits for the required electives (10 cr each) are distributed with the ratio 3:3:3:1 among
semesters 5-8.
B. Grundstudium, Fundamental Courses
Note: W - Winter semester / S - Summersemester
Experimental Physics I: Mechanics and Thermodynamics
In this course students will be shown how experimental experience of a phenomenon leads to quantitative
formulation of its underlying principles. This includes stating a scientific hypothesis, designing and
performing an appropriate experiment to test the hypothesis, analyzing the results, and developing a
mathematical expression and generalization for them.
Specific topics that are covered are the kinematics and dynamics of a point mass, two-body motion,
mechanical vibrations and waves, rigid bodies, non-rigid bodies, thermodynamics, and gas kinetics.
Particular emphasis is placed on the principles and methods which occur repeatedly in solutions of
physical problems, for example, Newton's equations of motion, the expression for a harmonic oscillator,
equations of motion for bodies in a collision, and also the steps of approximation in the development
of a physical model: point mass, rigid body, continuum, thermodynamic (phenomenological) and statistical
descriptions, the laws of thermodynamics. Applications to astro-, geo-, and particle physics, as well
as to chemical and thermal technologies (Wärmetechnik) are also discussed.
Problem sets and quizzes are offered in the accompanying discussion sections. Small study groups
("Arbeitsgemeinschaften") can be organized for students to perform further exercises on the physical and
mathematical principles discussed in the lecture.
Experimental Physics II: Electromagnetism and Optics
As in Experimental Physics I, the structure of this course follows the path taken between experimental
experience and quantitative formulations of fundamental laws, here, of electromagnetism and optics.
Specifically, the lectures cover electrostatics, stationary currents, magnetism, time-dependent fields,
time-dependent currents, electromagnetic oscillations and waves, geometrical optics, wave optics, and
optical instruments.
As in the first course, special emphasis is placed on the general methods and principles which
appear in many physical problems, for example, the definition and description of a field, analogies
and differences between electric and magnetic fields, long solenoids and plate capacitors, Maxwell's
equations, the description of waves, and typical wave phenomena. During the course, students will be
guided toward the "interface" of theoretical description and real applications in electrical devices,
electronics, optical devices and lasers.
In addition to the usual tutorial discussions, small study groups can be organized to allow
students to deepen their understanding of physical and mathematical aspects of the material.
Experimental Physics III: Atomic and Molecular Physics
What is an atom and how big is it? Is it possible to see individual atoms? Are electrons and photons
wave- or particle-like? What is the structure of an atom and how are molecules, clusters, and solids
formed from atoms? In these lectures these questions will be answered with the discoveries of
historically important, but also state-of-the-art experiments. We will discuss scattering experiments;
the interaction of atoms and molecules with light of various wavelengths; and the behavior of atoms
and molecules in electric and magnetic fields. Students may get the impression that this is merely
a summary of observations of various experiments. Indeed, it is not very easy to understand atomic
and molecular physics without an understanding of quantum mechanics. Nevertheless, it an important
goal of this course for students to be able to systematically interpret the observations of the
presented experiments and thus be led to the seminal results that will be treated again in the fourth
semester, in theoretical physics, on a formal mathematical basis. A not less important objective is
for students to become familiar with the modern experimental methods and analytical approaches that
led to the development of e.g., the laser or the tunneling microscope.
Key words and concepts: Atom, photon, free electron, characteristics of matter waves, hydrogen
atom, basics of quantum mechanics, optical transitions, multi-electron systems, periodic table of
the elements, atoms in electric and magnetic fields, structure of molecules, energy levels of diatomic
molecules, clusters - from atoms to solids.
Experimental Physics IV: Nuclei and Elementary Particles
What are exactly the fundamental building blocks of matter? What are atomic nuclei made of? What
are hadrons, leptons, and quarks? What are the fundamental interactions between these particles and
their anti-particles?
To answer these questions (as well as can be done with today's knowledge base), as in the course
on atomic and molecular physics, in this course we will also present the results of historic experiments
as well as the observations of current experiments in high-energy physics. But in addition to this
fascinating, virgin realm of physics (fundamental forces of nature), in this course students will
learn about nuclear stability, nuclear fission and fusion, radioactive decay, the interaction of alpha-,
beta-, and gamma-radiation with matter, dosimetry, and radiation shielding. This
course will cover nuclear magnetic resonance (nmr), the Mössbauer Effect, gamma-ray
spectroscopy, and collisions between electrons and positrons.
Key words and concepts: Properties of nuclei, nuclear stability and decay, radioactivity,
interaction of particles with matter, nuclear reactions, special experiments in nuclear physics,
radiation shielding, nuclear power plants, nuclear structure, scattering experiments, production of
subatomic particles, fundamental forces of nature, elementary particles.
Theoretical Physics I: Mechanics
Motion of a particle
- Fundamental expressions of space, time; relativity principle; Galilei and Lorentz transformations;
Newton's equations of motion, the integrability problem
- Motion in one dimension
Velocity dependent forces, space-dependent force and definition of a
potential, free fall, harmonic oscillator, frictional force, damped oscillator with and without a
harmonic driving force, Green's functions
- Vectorial representation of motion
Representation of curves and surfaces, motion of a point
through space, isotropic oscillator, trajectory of a projectile with friction, rotational motion,
charge in a homogeneous magnetic field
- Conservation laws
Conserved quantities, conservation of momentum, conservation of angular
momentum, conservation of energy, force as a potential gradient
- Motion in a central field
Polar coordinates, combination of the laws of conservation of
angular momentum and energy, bound states, planetary motion
Systems of particles
- Two-particle system
Equation of motion and interaction, conservation laws, separation of
center-of-mass and relative motions, elastic collisions
- Many-particle system
conservation laws, pair-wise interactions, transition to continuum
Advanced Mechanics
- Variational principle
Variation of trajectories, Lagrange functions of simple systems
- Phase space and the Hamiltonian description
Expression of motion in phase space, Hamiltonians,
canonical equations, time dependence, conserved quantities, Poisson brackets, and transition to
quantum mechanics
Theoretical Physics II: Quantum Mechanics
This course presents the fundamental theoretical concepts required for the description of microphysical
phenomena. We begin by presenting experimental results which cannot be explained by classical mechanics
alone and proceed, step-by-step, toward devising the mathematical apparatus of quantum mechanics. A
major portion of the class is devoted to the application of the Schrödinger equation to simple quantum
systems (locally constant potential, harmonic oscillator, hydrogen atom). Spin is discussed in the
context of the non-relativistic Pauli equation. Systems for which solution via the Schrödinger equation
is difficult, for example, multiple particle systems and collision processes, will be treated by various
approximation methods, e.g., the variational principle and perturbation theory. Students will learn that
these methods are the basis for attempting solutions of more complex problems, requiring more theoretical
sophistication.
Key words and concepts: Mathematical and physical fundamentals, Schrödinger equation, mathematical
apparatus of quantum mechanics, central field, spin, approximation methods, many-particle systems,
quantum theory of scattering.
Practicum in Physics I - IV
In the practicum, students learn to plan, construct, execute, and evaluate experiments. Basic
measurement and analysis procedures are learned, and first-hand experience of physical laws and
phenomena is gained, from selected laboratory exercises in mechanics, thermodynamics, optics,
electromagnetism, and atomic and molecular physics. Students should be able to deepen their understanding
of physics and of cause-and-effect relationships in physical processes.
Students are trained to work independently, maintain an exact laboratory record, and to critically
evaluate their measurements. They learn the basic ways of error analysis. To pass the course and obtain
a certificate "Physikalisches Grundpraktikum", 38 experiments must be completed.
C. Hauptstudium, Advanced Courses
Note: W - Winter semester / S - Summersemester
Physics of Condensed Matter I
In this course the properties of crystalline solids are described via the model of an infinite crystal.
In the adiabatic approximation the slow movement of the atoms is separated from the fast motion of the
electrons, thus allowing a sequential treatment of the vibronic, electronic, and finally the optical,
magnetic, and superconducting properties of solids. The course concludes with a discussion of effects
which appear for spatially limited solids, e.g., quantum wells, wires, and dots.
The discussion section will be primarily concerned with providing students with a feeling for the
dimensions of parameters typical for solids, for example, the bandgap energy of semiconductors in
relation to the thermal energy kT. This is of extreme importance when estimating the probability of
observing a physical effect under given experimental conditions.
Physics of Condensed Matter II
This course focuses on spectroscopic methods of characterizing solids, in particular, their surfaces.
Methods of sample preparation in ultra-high vacuum and sample growing methods, specifically, epitaxy,
are introduced. Various probes, e.g., electrons, heavy-particle beams, light, and their interactions
with solids are discussed, and the various information provided by each probing method are compared.
Electron spectroscopic methods, such as Auger and photoemission spectroscopy, and optical methods, such
as infrared and Raman, are presented along with electrical measurements. A summary of the newest
developments in scanning probe methods concludes the course.
In the discussion section the research apparatuses used at the Institute of Physics will be
demonstrated.
Atoms, Molecules, Lasers
- Atoms
- Electronic structure, selection rules, eigenvalues, hydrogen atoms, Schrödinger equation
- Molecules
- Chemical bonds, hydrogen molecule, (Hund-Mullikan variational approximation,
Heitler-London), Born-Oppenheimer approximation, Franck-Condon principle, vibrational states,
rotational states, reaction dynamics, magnetic properties
- Interaction between radiation and matter
- Linear response, nonlinear properties
- Laser/Maser
- Principle of operation (population inversion, rate equations, coherence), gas laser,
solid state laser, dye laser
- Modern spectroscopic methods
- Coherent methods, saturation spectroscopy, laser cooling of atoms,
magnetic resonance experiments, level-crossing experiments, molecular beams, cluster beams, wave packet
dynamics, fluorescence correlation
Selected Topics from Modern Physics
This series of lectures discusses current problems in solid state physics on a microscopic level.
Primarily metallic systems are discussed, however, some emphasis is placed on the metal-insulator
transition. Subsystems such as the static structure, the electronic system, dynamic structure, and
the connection and interaction between energy and momentum are presented. Typical changes that occur
in the transition from a disordered to a quasi-crystalline and single crystalline solid are treated.
Theoretical Physics III: Electrodynamics
- Electrostatic field:
- Sources = charges; fields of highly symmetric charge distributions; electrostatic potential;
multipole expansion; Poisson equation; image charges; theory of potentials
- Fields of inductance of stationary currents:
- Vortices = currents; current density and conservation of charge; Biot-Savart Law; vector potentials
- Dipoles and dipole layers:
- electrical and magnetic dipole moment; potentials and fields; torque and force in external
fields; Larmor precession
- Static fields in a medium:
- microscopic and macroscopic charges; currents and fields; oriented and induced atomic dipoles;
fields (E, D, P) and (B, H, M); material equations; boundary problems; magnetostatics
- Maxwell equations:
- Induction law and Lenz' law; electromotive force and frame of reference; self inductance and
mutual inductance; displacement current; Viererpotential; gauge conditions; delayed potentials
- Energy and momentum:
- field energy (self and interaction); the relation between potential energy and force; energy
density and energy flow density; Lorentz force; momentum density and the Maxwell tensor; interaction
between current loops
- Time-dependent electromagnetic fields:
- slowly moving charges; skin effect; wave equation and the Telegrafengleichung; phase velocity;
index of refraction; plane waves; spherical waves; electric dipole radiation (far-field and near-field);
radiation pressure
- Applications and mathematical tools:
- derivation of the field distribution from given charge, current, and material distributions;
energy and force; wave dynamics and scattering; vector potentials, sources, and vortices; line
integrals, surface integrals, volume integrals, integral theorems; Green's functions, partial
differential equations; spherical harmonics, cylindrical harmonics
Theoretical Physics IV: Statistics and Thermodynamics
- Thermodynamical description:
- Equilibrium, state variables, intensive variables, equations of state, model systems
- The Laws of Thermodynamics:
- the internal energy state variable, heat capacity, irreversibility, cyclic processes, the entropy
state variable, temperature, Clausius inequality, entropy near T = 0
Thermodynamic potential and conditions for equilibrium: Gibbs' law, chemical potential, maximum entropy
of a closed system, variational principle with constraints, phase equilibrium and phase transitions,
Clausius-Clapeyron equation, partial molar properties
Statistical ensembles: Distributions--binomial, Gaussian, Poisson; means, deviations and variance;
entropy and information; motion in phase space; Liouville theorem; Boltzmann factor; microcanonical and
canonical distributions; statistical operators; master equations; ergodicity
Microstates and thermodynamical state: Boltzmann's definition of entropy; state density and counting
of microstates; sum of states and state integrals; transition to thermodynamics
Equilibrium of a system in contact with a bath: Macrocanonical distribution and macrocanonical
formalism; equivalence of the canonical distribution in the thermodynamical limit
Applications: ideal gas; ideal gas and external fields; Van der Waals gas; virial expansion;
magnetic and dielectric media; special processes (adiabatic, Carnot, Joule-Thompson); kinetics and
transport processes (Maxwell distribution, diffusion, heat transport, Boltzmann equation); harmonic
oscillator; ideal quantum gases (fermions, bosons); black-body radiation; two-level systems; Ising model.
Theoretical Physics V: Continuum Physics
This course is an introduction to modern methods of describing non-rigid media using field theory, and
the fundamentals of the theory of elasticity and hydrodynamics. The course introduces kinematic tensors
such as for deformation and velocity gradients, and Cauchy's tensor for the shear modulus. Important
theoretical models for elongation, shear, torsion, and bending are discussed in their linearized
approximations (geometrical and physical linearization). In a treatment of hydrodynamics we deal with
the equations of motion for ideal and viscous fluids, from which the fundamental laws of fluid dynamics
are derived, such as those governing vortices and simple flow geometries. Advanced topics such as
properties of nonlinear materials and stability theory (critical point phenomena, turbulence) will
also be touched upon.
Keywords: Kinematics of deformable bodies, kinetics, theory of elasticity, hydrodynamics.
Theoretical Physics VI: Selected Topics from Modern Physics
- Thermal radiation
- Radiation field and photons
- Photon statistics
- Calculation of thermodynamic state variables
- Density of states and spectral distribution
- Quantum statistical description of time-dependent states
- The statistical operator and the von-Neumann equation
- Pure and mixed states
- State evolution in closed systems
- Time-dependence of average values and entropy
- Relaxation processes in macroscopic systems
- Quantum mechanical calculation of transition rates (golden rule)
- Energy exchange via external perturbations
- Energy conservation in internal perturbations, nonradiative processes
- The master equation and irreversibility
- Relaxing subsystem in a heat bath
- Relaxation of two- and more-level systems
- Electron-photon interactions
- Interactions of charged particles with electromagnetic fields
- Lagrange function of a radiation field and the Hamilton formalism
- Eigenvibrations and photon counts
- Induced emission and absorption processes
- Quantization of a radiation field, photon states and oscillator methods
- Rates of spontaneous and induced processes
- Dipole approximation, absorption cross sections, summation rules for oscillator strengths
Advanced Seminar
The "Oberseminar" provides students with a chance to prepare and present a lecture on a subject of
their choice, in the areas of solid state physics and materials science. The talk should be a report
on both the theoretical and experimental state-of-art. Students can choose among: fullerenes and
fullerides, Landauer-Büttiker theory, spin glasses, the glass transition, bulk metallic glasses
nanocrystalls, fractals, relaxations.
Physics Elective
Usually the elective course in physics is held over two semesters. The total number of semester hours
is flexible, and can be distributed among lecture and discussion sections. Each year, four different
electives are offered. The topics of each elective are chosen by each faculty member and are mentioned
in Section III C.
Non-Physics Elective
The non-physics electives are taught by the Institute of Chemistry and other departments. These
course span typically two semesters; a distribution of credit hours among lectures and discussion
sections is possible. Courses are offered in
- Economics
- Computer Science
- Chemistry/Physical Chemistry
- Mathematics
- Technical Thermodynamics (Chemical Engineering?)
- Fluid Mechanics
- Telecommunications technology/Microengineering
- Electronics/Microelectronics
Advanced Practicum I and II
In addition to gaining a familiarity with various areas of physics, the Advanced Practicum is intended
to introduce students to day-to-day laboratory work which requires developing an ability to grasp, on
short notice, new techniques and topics in physics, keeping an accurate record of the procedures and
experiments undertaken, critical analysis of the experimental results, and being able to write and
speak about them and defend them in a public forum. It is desired that students be allowed to work as
independently as possible. Particular emphasis is placed on the keeping of good laboratory records that
contain all the components of a scientific publication, and also of a Diplom thesis: an introduction,
experimental section, results, discussion, and summary.
Each student is required to give a 20 to 30 minute lecture about his or her experiment. This gives
them the opportunity to practice skills needed for a successful oral defense. In addition 24 other
experiments must be tried and will be listed in the "Testat" certificate.
Laboratory Practicum I: Overview ( Runaround Practicum)
In this practicum, the experiments are conducted on the research apparatuses of each professor's
laboratory. As such, the procedures are based on problems that are within a field of interest of the
Institute faculty. This allows students to acquaint themselves with the Institute's offerings and to
begin deciding on their own area of specialization for their Diplom thesis. Students are allowed to
choose freely among the experimental setups; however, it is required that each experiment be done in a
different faculty member's laboratory and that at least one experiment be in theoretical physics.
In order to emphasize the link between theoretical and experimental physics, the instructors of the
theoretical physics courses will provide students with exercises that are relevant to the experimental
areas of research of the department.
As in the Advanced Practicum, students are expected to keep accurate laboratory notebooks and will
also be given 20 to 30 minutes for an oral presentation to explain and defend their work on one
experiment of their choice. A total of seven experiments must be performed; each will be certified
with a "Testat".
Laboratory Practicum II: Specialization
During this semester, students work in a research group of their choice on a project that is usually
on the cutting edge of physics. The project may be on the same subject as the Diplom thesis that begins
in the subsequent semester. However, the choice of this Practicum's topic and that of the Diplom topic
are completely independent of one another. The time in which the Practicum project should be completed
is agreed upon by the student and the project advisor.
The results of this Practicum project must be reported in written form, in a word-processed document,
and also in a 20- to 30-minute oral presentation.





(©) L. Feige (12/1997)