Electrocatalytic Oxygen Reduction
at Transition Metal Porphyrin Modified Carbon Electrodes
Figure 1: Structures of investigated porphyrins (TMPP = 5,10,15,20-Tetrakis-(p-Methoxyphenylporphyrin), TPP = 5,10,15,20-Tetraphenylporphyrin, TPyP = 5,10,15,20-Tetra-(4-N)pyridylporphyrin)
On the subject
The purpose of this study is to investigate the reduction of molecular dioxygen at metal porphyrin modified carbon electrodes.
It covers metalloporphyrins of group VIIIA elements. Substituents with variable electronic configuration at the porphyrin ring will provide a deeper insight into the mechanism of dioxygen reduction in acidic aqueous media.
Attention is paid to mechanistic and kinetic investigations. Depending primarily on the electronic state and some steric aspects, the mechanism of dioxygen coordination and reduction as well as the catalytic activity of particular porphyrins are investigated. The 4-electron reduction is of special interest.
Electrochemical and spectroelectrochemical methods are applied, especially ring disk electrodes, surface Raman and IR spectroscopy, and electrochemical impedance measurements. Using imaging techniques like atomic force microscopy or scanning electron microscopy is considered.
Aim of the study
The electrochemical reduction of dioxygen is a reaction of great importance in energy conversion and sensor technology. With the introduction of fuel cells the demand for a convenient oxygen reduction electrode arose. The best known catalyst for oxygen reduction up to now - metallic platinum - turned out to be disadvantageous: It is expensive and has still a high oxygen reduction overpotential.
The search for alternatives led - among other catalysts - to chelates of transition metals, especially to group VIIIA metal porphyrins and phthalocyanins. At this point the present study is located. The expected results related to the influence of substituents, the type of dioxygen coordinaton, and the electrochemical behaviour shall help to develop and optimize these catalysts more effectively in the future.
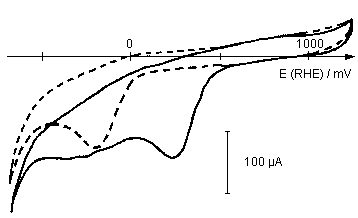 Figure 2: Effect of porphyrin modification of a graphite electrode on oxygen reduction (CV of TPyP on pyrolytic graphite, 100 mV / s in O2-saturated 1 M HClO4 solution). Dotted line: pure graphite, normal line: FeTPyP on graphite. The cathodic overpotential is reduced by approximately 500 mV.
Figure 2: Effect of porphyrin modification of a graphite electrode on oxygen reduction (CV of TPyP on pyrolytic graphite, 100 mV / s in O2-saturated 1 M HClO4 solution). Dotted line: pure graphite, normal line: FeTPyP on graphite. The cathodic overpotential is reduced by approximately 500 mV.
The methods
A first screening of potential catalysts can be performed very easily with cyclic voltammetry. Regardless the relatively simple setup, the two main parameters can be estimated: overpotential and catalytic activity towards dioxygen reduction.
A decision whether two- or four-electron reduction happens can be made with rotating ring disk electrodes. Such parameters can be taken from Koutecki-Levich plots as well as from the occurence of reducible H2O2 at the ring.
Vibrational spectroscopic investigations at the electrode/solution interface can provide information about the type of chelate coordination to the electrode and the type of dioxygen coordination to the metal ion.
Kinetic results can be verified and extended by means of electrochemical impedance measurements, especially concerning type and number of involved steps and possible further inhibitions, like diffusion.
Finally, the size of the molecules makes it possible to apply imaging techniques to investigate the geometry of chelate coordination to the surface.
A heat treatment is not planned.