High performance Li-rich cathode materials for lithium ion batteries
Introduction:
Conventional lithium ion batteries materials (such as LiCoO2, LiFePO4, LiMn1/3Ni1/3Co1/3O2, etc.) are unable to meet the requirements of the energy supply of EVs due to limited capacities of the presently commercially used cathodes (<200 mAh/g), thus, the development of green, low-cost and high performance cathode materials for the next generation LIBs is significant. Li-rich lithium ion cathode materials (Li2MnO3-LiMO2, M=Ni,Co,Mn) have become appealing, because they exhibit much higher capacities(~250mAh/g). However, there are still major issues that need to be addressed before these compounds can be considered as high-energy cathodes for Li-ion batteries, especially for electric vehicle application. Notable among of them are poor rate capability, high first cycle irreversibility and significant decrease in the discharge voltage plateau with successive cycling.
To face these problems, several strategies have been attempted to improve the performance of lithium rich materials, for instance, reducing the particle size of the materials to nano-scale to improve the rate performance, surface modification with electrochemical inactive oxides (Al2O3, AlPO4, SiO2, ZnO) or the electrochemically active (CoPO4 and MnOx) to enhance the initial coulombic efficiency and the cycleability performance of the lithium rich materials.
Research plan:
Through sol-gel method to synthesize nano-size li-rich cathode materials, controlling the experiment parameters to synthesize metal oxide compounds or high ionic conductance coating on the surface of host materials to improve the performance of lithium ion battery.
Through hydrothermal method to synthesize nanocomposite of li-rich cathode materials, which combine with high conductivity materials like CNT or graphene to improve the performance of materials for lithium ion batteries.
Through XRD, SEM, TEM, EDS to characterize the structure and morphology of the materials and GITT, CV, EIS etc electrochemical methods to understand the mechanism of the electrochemical process.
 |
Fig. 1. Initial charge-discharge curves of AlF3 coating Li1.2Ni0.13Co0.13Mn0.54O2
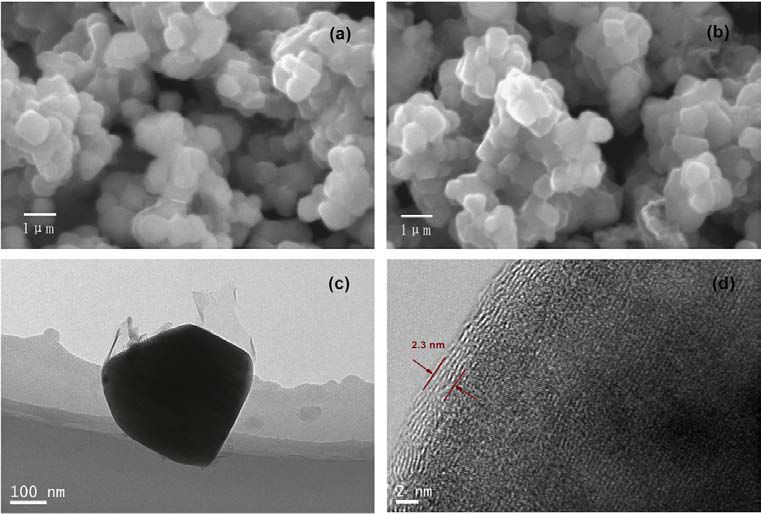 |
Fig. 2. (a) SEM image of the pristine Li[Li0.2Mn0.54Ni0.13Co0.13]O2 particle,
(b)SEM image of the graphene-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 particle,
(c)TEM image of the graphene-coated particle, and(d)HRTEM image of the graphene-coated particle.
References:
[1] Sun-Ho-Kang etc, Enhancing the rate capability of high capacity xLi2MnO3.(1-x)LiMO2 (M = Mn, Ni, Co) electrodes by Li–Ni–PO4 treatment.Electrochemistry communication 11(2009) 748-751.
[2] Min-Sik Park etc, On the surface modifications of high-voltage oxide cathodes for lithium-ion batteries: new insight and significant safety improvement.J.Mater.Chem.,2010,20,7208-7213.
[3] Seung-Taek Myung, etc. Effect of AlF3 Coating on Thermal Behavior of ChemicallyDelithiated Li0.35[Ni1/3Co1/3Mn1/3]O2. J.Phys.Chem.C 2010,114,4710-4718.
[4] Stefan Klink, etc. Tailoring of CNT surface oxygen groups by gas-phase oxidation and its implications for lithium ion batteries. 2012,15,10-13.
[5] Xiaoguang Hao,etc. Two-step hydrothermal synthesis of submicron Li1+xNi0.5Mn1.5O4.ä for lithium-ion battery cathodes (x = 0.02, ä = 0.12), Dalton Trans., 2012,41,8067-8076.
[6] R.Santhanam,etc. Influence of lithium content on high rate cycleability of layered Li1+xNi0.30Co0.30Mn0.40O2 cathodes for high power lithium-ion batteries.,Journal of Power Sources., 2010, 195,7391-7396.
[7] Yang-Kook Sun,etc. The Role of AlF3 coatings in improving electrochemical cycling of Li-Enriched Nickel-Manganese Oxide Electrodes for Li-Ion Batteries., Adv.Mater. 2012, 24, 1192-1196.
[8] L.J.Fu., etc. Electrode materials for lithium secondary batteries prepared by sol–gel methods., Progress in Materials Science, 2005, 50, 881-928.
