Electrochemical breakdown of lignin
Lignin is a primary constituent of wood together with cellulose and hemicelluloses. The biopolymer lignin is one of the most abundant renewable feedstocks in the world [1-3]. Moreover, lignin represents the largest source of aromatic compounds among renewables and can be considered as non-food biomass. It usually occurs as a major waste fraction of the pulping industry on a multimillion ton scale [4]. This source has the potential to be an alternative for petroleum-based production of fuels as well as fine chemicals [5-7].
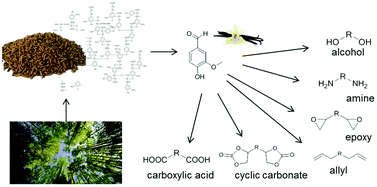
In the last few decades, the large amount of aromatic structural features making up the polymer led to much effort concerning efficient breakdown methods yielding high value fine chemicals like vanillin, acetovanillone and guaiacol.
Electrochemistry is one of the most promising approaches for breakdown of lignin into valuable aromatic compound.
• only electrons serve as reagent• very mild reaction conditions
• green chemistry
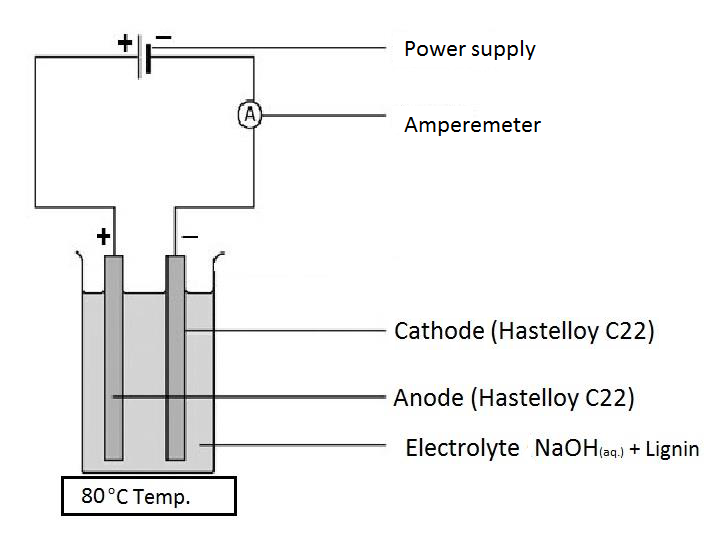
The oxidation of lignin usually results in the conversion of phenolic nuclei to o- or p-quinonoid structures or in the rupture of the aromatics leading to enoic or dienoic acids. Vanillin is usually obtained from the cleavage of the propanoid side chain by electrolysis.
REFERENCES:
- Chatel, G.; Rogers, R. D. ACS Sustainable Chem. Eng. 2014, 2, 322–339.
- Hanson, S. K.; Baker, R. T.; Gordon, J. C.; Scott, B. L.; Sutton, A. D.; Thorn, D. L. J. Am. Chem. Soc. 2009, 131, 428–429.
- Türk, O. Stoffliche Nutzung nachwachsender Rohstoffe; Springer: Wiesbaden, Germany, 2014.
- Chakar, F. S.; Ragauskas, A. J. Ind. Crops Prod. 2004, 20, 131–141.
- Yáñez, M.; Rojas, J.; Castro, J.; Ragauskas, A. J.; Baeza, J.; Freer, J. J. Chem. Technol. Biotechnol. 2013, 88, 39–48.
- Zhang, W.; Chen, J.; Liu, R.; Wang, S.; Chen, L.; Li, K. ACS Sustainable Chem. Eng. 2014, 2, 683–691.
- Lange, H.; Decina, S.; Crestini, C. Eur. Polym. J. 2013, 49, 1151–1173.
