|
ELECTROCHEMICAL OXIDATION OF CHLORINATED HAZARDOUS ORGANICS IN WASTEWATER ON BORON - DOPED DIAMOND ELECTRODE (BDD)
1. Background and cell design
The chlorinated organic compounds comprise a large group of compounds with a complex diversity of biologic effects[1]. Some of chlorinated organic compounds having a concerned risky impact to environment and human body are dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs) and dioxin, their structural formulas are presented in Fig 1[2].
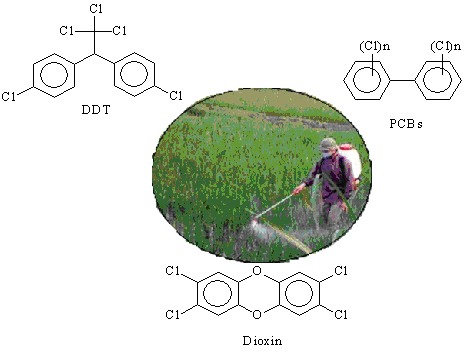
Fig 1. Structural formulas of some chlorinated hazardous organics
Highly doped diamond films as anodes are recent candidates for chlorinated organic pollutants electrooxidation. Diamond films are grown on subtrates Nb, Ta,W or simply steel using the hot filament chemical vapour deposition (HF-CVD) technique[3-6]. The conductivity of diamond is improved by doping with boron. Boron doping is usually achieved by adding B2H6[7,8] or B(OCH3)3[9,10] into the gas phase during deposition.
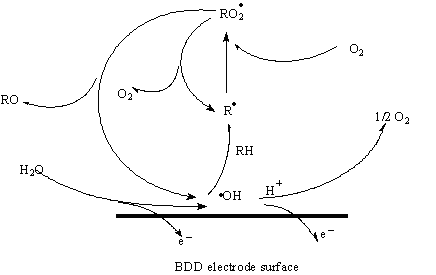
Fig 2. Diamond surface and electrochemical process
Electrooxidation (which is simplified in Fig 2) can destroy chlorinated organic compounds in wastewater by generating OH*-radical,s which will oxidize chlorinated hazardous organics to end products: CO2, H2O, Cl2, ect...
2. Oxidation mechanism
Water is first discharged at the anode active sites M, producing hydroxyl species ( either adsorbed species, if the potential is low or, if the potential is high enough, even free radicals)[11]:
M + H2O → M (HO•) + H+ + e-
The electrogenerated hydroxyl species are involved in the mineralization of chlorinated pollutants R[11]:
R + M (HO•) → M + mineralization products + H+ + e-
References:
- Annika Hanberg, Toxicology of environmentally persistent chlorinated organic compounds.Pure & Appl.Chem., Vol.68, No .9,pp. 1791 – 1799, 1996.
- Available online: http://www.pure-earth.com/chlorine.html
- J. Asmussen, D.K. Reinhard, Diamond Film Handbook, Michigan State University, East Lansing, MI, 2002.
- S. Matsumoto, Y. Sato, M. Kakmo, N. Setaka, Growth of diamond particles from methane–hydrogen gas, J. Mater. Sci. 17 (1982) 3106–3112.
- S. Matsumoto, Y. Sato, M. Kakmo, N. Setaka, Vapor deposition of diamond particles from methane, Jpn. J. Appl. Phys. 2 (1982) L183–L185.
- P.K. Bachmann, Microwave plasma CVD and related techniques for low pressure diamond synthesis, in: A. Lettington, J.W. Steeds (Eds.), Thin Film Diamond, London, Chapman & Hall, 1994, pp. 31–53.
- J. Mort, D. Kuhman, M. Machonkin, M. Morgan, F. Jansen, K. Okumura, Y.M. Legrice, R.J. Nemanich, Boron doping of diamond thin-films, Appl. Phys. Let. 55 (11) (1989) 1121–1123.
- N. Fujimori, H. Nakahata, T. Imai, Properties of boron- doped epitaxial diamond films, Jpn. J. Appl. Phys. 29 (1990)824–827.
- J.G. Ran, C.Q. Zheng, J. Ren, S.M. Hong, Properties and texture of B-doped diamond films as thermal sensor, Diam. Relat. Mater. 2 (1993) 793–796.
- M. Fryda, D. Herrmann, L. Schafer, C.P. Klages, A. Perret, W. Haenni, Ch. Comninellis, D. Gandini, Properties of diamond electrodes for wastewater treatment, New Diam. Front. C. Technol. 9 (1999) 229–240.
- Synthetic Diamond films. Enric Brillas and Carlos Alberto Martinez - Huitle
|
